Products Category
- FM Transmitter
- 0-50w 50w-1000w 2kw-10kw 10kw+
- TV Transmitter
- 0-50w 50-1kw 2kw-10kw
- FM Antenna
- TV Antenna
- Antenna Accessory
- Cable Connector Power Splitter Dummy Load
- RF Transistor
- Power Supply
- Audio Equipments
- DTV Front End Equipment
- Link System
- STL system Microwave Link system
- FM Radio
- Power Meter
- Other Products
- Special for Coronavirus
Products Tags
Fmuser Sites
- es.fmuser.net
- it.fmuser.net
- fr.fmuser.net
- de.fmuser.net
- af.fmuser.net ->Afrikaans
- sq.fmuser.net ->Albanian
- ar.fmuser.net ->Arabic
- hy.fmuser.net ->Armenian
- az.fmuser.net ->Azerbaijani
- eu.fmuser.net ->Basque
- be.fmuser.net ->Belarusian
- bg.fmuser.net ->Bulgarian
- ca.fmuser.net ->Catalan
- zh-CN.fmuser.net ->Chinese (Simplified)
- zh-TW.fmuser.net ->Chinese (Traditional)
- hr.fmuser.net ->Croatian
- cs.fmuser.net ->Czech
- da.fmuser.net ->Danish
- nl.fmuser.net ->Dutch
- et.fmuser.net ->Estonian
- tl.fmuser.net ->Filipino
- fi.fmuser.net ->Finnish
- fr.fmuser.net ->French
- gl.fmuser.net ->Galician
- ka.fmuser.net ->Georgian
- de.fmuser.net ->German
- el.fmuser.net ->Greek
- ht.fmuser.net ->Haitian Creole
- iw.fmuser.net ->Hebrew
- hi.fmuser.net ->Hindi
- hu.fmuser.net ->Hungarian
- is.fmuser.net ->Icelandic
- id.fmuser.net ->Indonesian
- ga.fmuser.net ->Irish
- it.fmuser.net ->Italian
- ja.fmuser.net ->Japanese
- ko.fmuser.net ->Korean
- lv.fmuser.net ->Latvian
- lt.fmuser.net ->Lithuanian
- mk.fmuser.net ->Macedonian
- ms.fmuser.net ->Malay
- mt.fmuser.net ->Maltese
- no.fmuser.net ->Norwegian
- fa.fmuser.net ->Persian
- pl.fmuser.net ->Polish
- pt.fmuser.net ->Portuguese
- ro.fmuser.net ->Romanian
- ru.fmuser.net ->Russian
- sr.fmuser.net ->Serbian
- sk.fmuser.net ->Slovak
- sl.fmuser.net ->Slovenian
- es.fmuser.net ->Spanish
- sw.fmuser.net ->Swahili
- sv.fmuser.net ->Swedish
- th.fmuser.net ->Thai
- tr.fmuser.net ->Turkish
- uk.fmuser.net ->Ukrainian
- ur.fmuser.net ->Urdu
- vi.fmuser.net ->Vietnamese
- cy.fmuser.net ->Welsh
- yi.fmuser.net ->Yiddish
[Coronavirus Pneumonia] Vaccination for the First Acupuncture in China Enters Clinical Trials
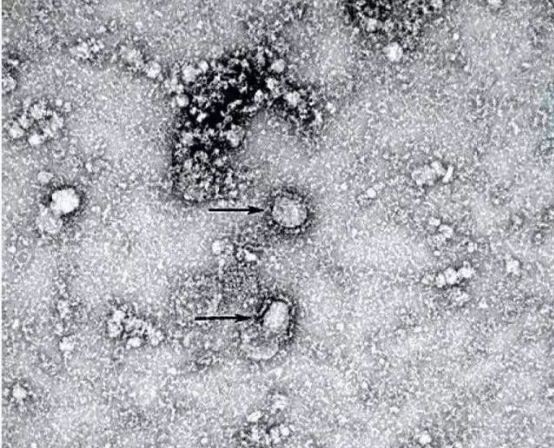
In response to the new crown pneumonia epidemic, China has begun a phase I clinical trial of the vaccine, with a total of 108 volunteers already receiving injections.
According to reports from China ’s “Science and Technology Daily” and “21st Century Business Herald” on Saturday, the first batch of volunteers participated in the trial is called “Phase I clinical trial of recombinant new coronavirus (2019-COV) vaccine (adenovirus vector)” .
The report stated that the volunteers participating in the clinical trial were divided into 3 groups of low dose, medium dose and high dose, 36 people in each group; the clinical trial time was from 16th to 31st of this month.
Designed to test vaccine safety
According to public information from the China Clinical Trials Registry, the trial is sponsored by the Institute of Bioengineering of the Academy of Medical Sciences of the Academy of Military Sciences and Kangsino Biological Co., Ltd. The trial is designed to test and evaluate the safety and effectiveness of the recombinant neocoronavirus vaccine.
The above vaccine is constructed by genetic engineering method, using replication-defective human type 5 adenovirus as a vector, and can express a novel coronavirus S antigen.
Chen Wei, a member of the Chinese Academy of Engineering who is responsible for this clinical experiment and a researcher at the Military Medical Research Institute, explained, "The principle is to 'surge' the virus on the premise of 'learning' the virus, and using flowers and trees to transform the one we need. Carrier virus, and injected into the body to produce immunity. "
There are not many volunteers required for the first phase of the trial. They are limited to permanent residents in Wuhan, and household registration residents in Wuchang, Hongshan, and Donghu Scenic Areas are preferred, aged 18 to 60.
A volunteer who received the vaccine on Thursday (19th) said that most of the volunteers who were vaccinated together were over 30 years old, mostly 40-50 years old. After screening and physical examination, eligible volunteers can be vaccinated. The next 14 days are the observation period for centralized isolation.
Earlier, Wang Junzhi, an academician of the Chinese Academy of Engineering, said at a press conference that China has already developed units that have made rapid progress in submitting clinical trial application materials to the China Food and Drug Administration, and has started relevant clinical trial program demonstration and recruitment of volunteers.
How long can it be put into practical use?
China: 6 months minimum
The first vaccine approved for clinical trials in China was developed by a scientific research team led by Academician Chen Wei, Academy of Military Medical Research, Academy of Military Sciences. It is a recombinant vaccine using adenovirus as a vector. At 20:18 on March 16, their new recombinant crown vaccine was approved to start clinical trials! In an interview with CCTV, Chen Wei said: "Vaccines are the most powerful technology weapon to end the new crown epidemic. We have done preliminary preparations for safe, effective, controllable quality, and large-scale production in accordance with international norms and domestic regulations. We are ready to start a formal clinical trial at any time. "
Clinical trials start, how far away is the vaccine from the market?
"Even with special events, vaccines have to go through several clinical trials, including Phase I, Phase II, and Phase III." Said Wu Zunyou, chief expert of epidemiology at the Chinese Center for Disease Control and Prevention. The trial finally concluded that if the vaccine is effective, the shortest estimate is also six months.
It turned out that the safety test for the first phase of clinical trials depends on the number of immunizations, even if the minimum time for a single session is not less than 20 days; the procedures and procedures for testing the clinical vaccination in the second phase of clinical trials require about 200 to 300 people. One month; clinical phase III is to evaluate the effectiveness of the vaccine. If the incidence of the patient is relatively high, even if a control group is needed, the sample size will be less and the time will be shorter, but the shortest time is 3 to 5 months. Not waiting.
It seems that for the Chinese, even if the first clinical trial of the new crown vaccine is successful and eventually proved effective, it will take at least half a year, that is, September this year before it can be put on the market.
"Vaccines are the most fundamental solution to new crown pneumonia. No matter which country makes the vaccine, it cannot be supplied to the whole world. This requires learning from each other, and after making it, we can learn from each other's strengths. It may take many manufacturers to produce it to supply the world. On November 18, Zhong Nanshan, an academician of the Chinese Academy of Engineering and leader of the high-level expert group of the National Health and Medical Commission, also answered questions from media reporters, "China's vaccine is developing very fast and will not be much worse than the United States. The United States is said to be available in September In humans, China is also racing, and it is estimated that it will not be much worse before and after. "
If you need any Epidemic Prevention Products, please feel free to contact us: [email protected]
You may also like:
More than 90%-total treat effective rate of New Coronavirus - Qingfei Paidu Decoction
FMUSER N95/KN95 Anti-virus Mask
FMUSER Alcohol spray 60ml /100ml/350ml
FMUSER infrared thermal imaging camera

