Products Category
- FM Transmitter
- 0-50w 50w-1000w 2kw-10kw 10kw+
- TV Transmitter
- 0-50w 50-1kw 2kw-10kw
- FM Antenna
- TV Antenna
- Antenna Accessory
- Cable Connector Power Splitter Dummy Load
- RF Transistor
- Power Supply
- Audio Equipments
- DTV Front End Equipment
- Link System
- STL system Microwave Link system
- FM Radio
- Power Meter
- Other Products
- Special for Coronavirus
Products Tags
Fmuser Sites
- es.fmuser.net
- it.fmuser.net
- fr.fmuser.net
- de.fmuser.net
- af.fmuser.net ->Afrikaans
- sq.fmuser.net ->Albanian
- ar.fmuser.net ->Arabic
- hy.fmuser.net ->Armenian
- az.fmuser.net ->Azerbaijani
- eu.fmuser.net ->Basque
- be.fmuser.net ->Belarusian
- bg.fmuser.net ->Bulgarian
- ca.fmuser.net ->Catalan
- zh-CN.fmuser.net ->Chinese (Simplified)
- zh-TW.fmuser.net ->Chinese (Traditional)
- hr.fmuser.net ->Croatian
- cs.fmuser.net ->Czech
- da.fmuser.net ->Danish
- nl.fmuser.net ->Dutch
- et.fmuser.net ->Estonian
- tl.fmuser.net ->Filipino
- fi.fmuser.net ->Finnish
- fr.fmuser.net ->French
- gl.fmuser.net ->Galician
- ka.fmuser.net ->Georgian
- de.fmuser.net ->German
- el.fmuser.net ->Greek
- ht.fmuser.net ->Haitian Creole
- iw.fmuser.net ->Hebrew
- hi.fmuser.net ->Hindi
- hu.fmuser.net ->Hungarian
- is.fmuser.net ->Icelandic
- id.fmuser.net ->Indonesian
- ga.fmuser.net ->Irish
- it.fmuser.net ->Italian
- ja.fmuser.net ->Japanese
- ko.fmuser.net ->Korean
- lv.fmuser.net ->Latvian
- lt.fmuser.net ->Lithuanian
- mk.fmuser.net ->Macedonian
- ms.fmuser.net ->Malay
- mt.fmuser.net ->Maltese
- no.fmuser.net ->Norwegian
- fa.fmuser.net ->Persian
- pl.fmuser.net ->Polish
- pt.fmuser.net ->Portuguese
- ro.fmuser.net ->Romanian
- ru.fmuser.net ->Russian
- sr.fmuser.net ->Serbian
- sk.fmuser.net ->Slovak
- sl.fmuser.net ->Slovenian
- es.fmuser.net ->Spanish
- sw.fmuser.net ->Swahili
- sv.fmuser.net ->Swedish
- th.fmuser.net ->Thai
- tr.fmuser.net ->Turkish
- uk.fmuser.net ->Ukrainian
- ur.fmuser.net ->Urdu
- vi.fmuser.net ->Vietnamese
- cy.fmuser.net ->Welsh
- yi.fmuser.net ->Yiddish
Handbook of COVID-19 Prevention and Treatment



Foreword
This is an unprecedented global war, and mankind is facing the same enemy, the novel coronavirus. And the first battlefield is the hospital where our soldiers are the medical workers. To ensure that this war can be won, we must first make sure that our medical staff is guaranteed sufficient resources, including experience and technologies. Also, we need to make sure that the hospital is the battleground where we eliminate the virus, not where the virus defeats us. Therefore, the Jack Ma Foundation and Alibaba Foundation have convened a group of medical experts who have just returned from the frontlines of fighting the pandemic. With the support of The First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU), they quickly published a guidebook on the clinical experience of how to treat this new coronavirus. The treatment guide offers advice and reference against the pandemic for medical staff around the world who are about to join the war. My special thanks goes out to the medical staff from FAHZU. While taking huge risks in treating COVID-19 patients, they recorded their daily experience which is reflected in this Handbook. Over the past so days, 104 confirmed patients have been admitted to FAHZU, including 78 severe and critically ill ones. Thanks to the pioneering efforts of medical staff and the application of new technologies, to date, we have witnessed a miracle. No staff were infected, and there were no missed diagnosis or patient deaths. Today, with the spread of the pandemic, these experiences are the most valuable sources of information and the most important weapon for medical workers on the frontline. This is a brand-new disease, and China was the first to suffer from the pandemic. Isolation, diagnosis, treatment, protective measures, and rehabilitation have all started from scratch. WE hope that this Handbook can provide doctors and nurses in other affected areas valuable information so they don't have to enter the battlefield alone. This pandemic is a common challenge faced by mankind in the age of globalization. At this moment, sharing resources, experiences and lessons, regardless of who you are, is our only chance to win. The real remedy for this pandemic is not isolation, but cooperation. This war has just begun.

Part One Prevention and Control Management
I. Isolation Area Management
1. Fever Clinic
1.1 Layout
(1) Healthcare facilities shall set up a relatively independent fever clinic including an exclusive one-way passage at the entrance of the hospital with a visible sign;
(2) The movement of people shall follow the principle of "three zones and two passages" : a contaminated zone, a potentially contaminated zone and a clean zone provided and clearly demarcated, and two buffer zones between the contaminated zone and the potentially contaminated zone;
(3) An independent passage shall be equipped for contaminated items; set up a visual region for one-way delivery of items from an office area (potentially contaminated zone) to an isolation ward (contaminated zone);
(4) Appropriate procedures shall be standardized for medical personnel to put on and take off their protective equipment. Make flowcharts of different zones, provide full-length mirrors and observe the walking routes strictly; (5) Infection prevention and control technicians shall be assigned to supervise the medical personnel on putting on and removing protective equipment so as to prevent contamination;
(6) All items in the contaminated zone that have not been disinfected shall not be removed.
1.2 Zone Arrangement
(1) Set up an independent examination room, a laboratory, an observation room, and a resuscitation room;
(2) Set up a pre-examination and triage area to perform preliminary screening of patients;
(3) Separate diagnosis and treatment zones: those patients with an epidemiological history and fever and/or respiratory symptoms shall be guided into a suspected COVID-19 patient zone; those patients with regular fever but no clear epidemiological history shall be guided into a regular fever patient zone.
1.3 Patient Management
(1) Patients with fevers must wear medical surgical masks;
(2) Only patients are allowed to enter the waiting area in order to avoid overcrowding;
(3) The duration of the patient's visit shall be minimized so as to avoid cross infections;
(4) Educate patients and their families about early identification of symptoms and essential preventative actions.
1.4 Screening, Admission and Exclusion
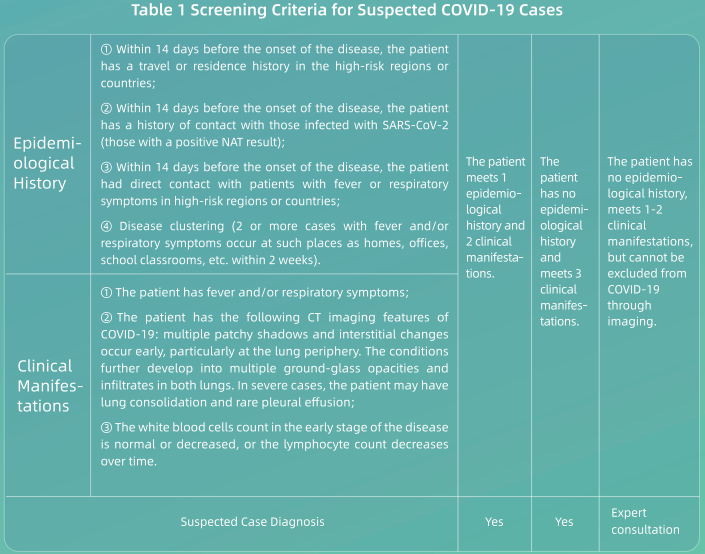
(1) All healthcare workers shall fully understand the epidemiological and clinical features of COVID-19 and screen patients in accordance with the screening criteria below (see Table 1 );
(2) Nucleic acid testing (NAT) shall be conducted on those patients who meet the screening criteria for suspected patients;
(3) Patients who do not meet the screening criteria above, if they do not have a confirmed epidemiological history, but cannot be ruled out from having COVID-19 based on their symptoms, especially through imaging, are recommended for further evaluation and to obtain a comprehensive diagnosis;
(4) Any patient who tests negative shall be re-tested 24 hours later. If a patient has two negative NAT results and negative clinical manifestations, then he or she can be ruled out from having COVID-19 and discharged from the hospital. If those patients cannot be ruled out from having COVID-19 infections based on their clinical manifestations, they shall be subjected to additional NAT tests every 24 hours until they are excluded or confirmed;
(5) Those confirmed cases with a positive NAT result shall be admitted and treated collectively based on the severity of their conditions (the general isolation ward or isolated ICU).
2. Isolation Ward Area
2.1 Scope of Application
The isolation ward area includes an observation ward area, isolation wards, and an isolation ICU area. The building layout and workflow shall meet the relevant requirements of the hospital isolation technical regulations. Medical providers with negative pressure rooms shall implement standardized management in accordance with relevant requirements. Strictly limit access to isolation wards.
2.2 Layout
Please refer to fever clinic.
2.3 Ward Requirements
(1) Suspected and confirmed patients shall be separated in different ward areas;
(2) Suspected patients shall be isolated in separated single rooms. Each room shall be equipped with facilities such as a private bathroom and the patient's activity should be confined to the isolation ward;
(3) Confirmed patients can be arranged in the same room with bed spacing of not less than 1.2 meters (appx 4 feet). The room shall be equipped with facilities such as a bathroom and the patient's activity must be confined to the isolation ward.
2.4 Patient Management
(1) Family visits and nursing shall be declined. Patients should be allowed to have their electronic communication devices to facilitate interactions with loved ones;
(2) Educate patients to help them prevent further spread of C0VID-19, and provide instructions on how to wear surgical masks, proper hand washing, cough etiquette, medical observation and home quarantine.
I.Staff Management
1. Workflow Management
(1) Before working in a fever clinic and isolation ward, the staff must undergo strict training and examinations to ensure that they know how to put on and remove personal protective equipment. They must pass such examinations before being allowed to work in these wards.
(2) The staff should be divided into different teams. Each team should be limited to a maximum of 4 hours of working in an isolation ward. The teams shall work in the isolation wards (contaminated zones) at different times. (3) Arrange treatment, examination and disinfection for each team as a group to reduce the frequency of staff moving in and out of the isolation wards.
(4) Before going off duty, staff must wash themselves and conduct necessary personal hygiene regimens to prevent possible infection of their respiratory tracts and mucosa.
2. Health Management
(1) The front-line staff in the isolation areas - including healthcare personnel, medical technicians and property & logistics personnel - shall live in an isolation accommodation and shall not go out without permission.
(2) A nutritious diet shall be provided to improve the immunity of medical personnel.
(3) Monitor and record the health status of all staff on the job, and conduct health monitoring for front-line staff, including monitoring body temperature and respiratory symptoms; help address any psychological and physiological problems that arise with relevant experts.
(4) If the staffs have any relevant symptoms such as fever, they shall be isolated immediately and screened with an NAT.
(5) When the front-line staff including healthcare personnel, medical technicians and property & logistics personnel finish their work in the isolation area and are returning to normal life, they shall first be NAT tested for SARS-CoV-2. If negative, they shall be isolated collectively at a specified area for 14 days before being discharged from medical observation.
Ill. COVID-19 Related Personal Protection Management
Notes:
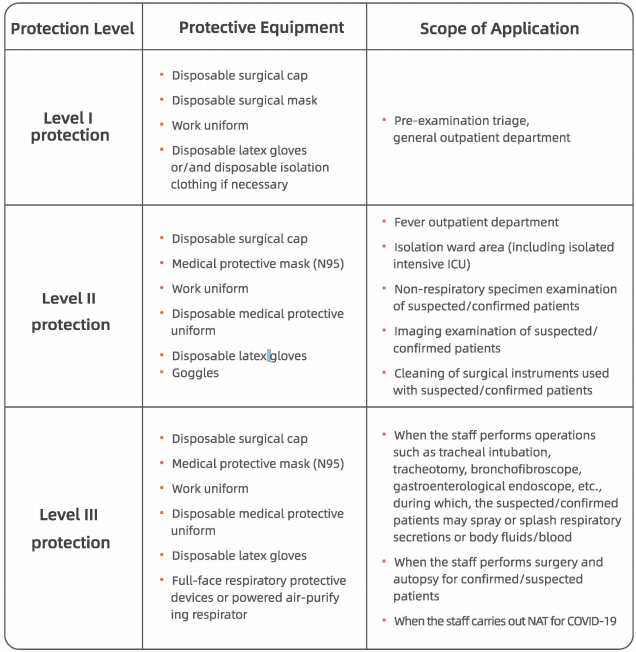
1. All staff at the healthcare facilities must wear medical surgical masks;
2. All staff working in the emergency department, outpatient department of infectious diseases, outpatient department of respiratory care, department of stomatology or endoscopic examination room (such as gastrointestinal endoscopy, bronchofibroscopy, laryngoscopy, etc.) must upgrade their surgical masks to medical protective masks {N95) based on Level I protection;
3. Staff must wear a protective face screen based on Level II protection while collecting respiratory specimens from suspected/confirmed patients.
IV. Hospital Practice Protocols during COVID-19 Epidemic
1. Guidance on Donning and Removing Personal Protective Equipment (P.P.E).to manage COVID-19 Patients
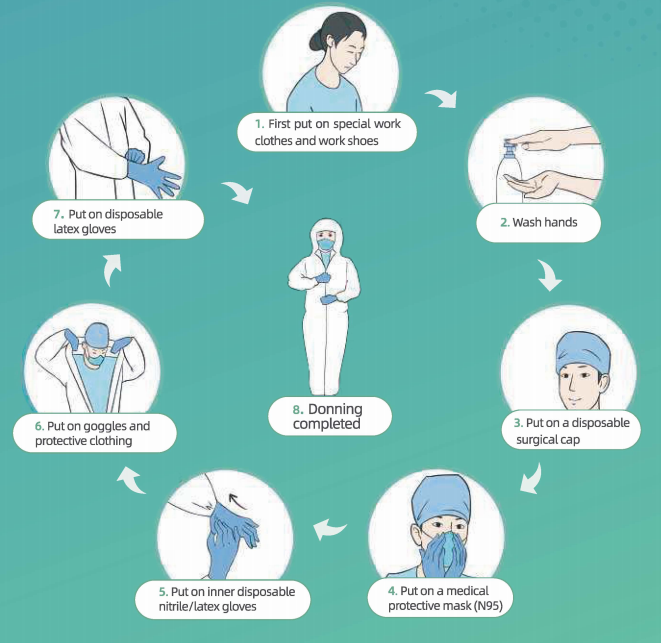
Protocol for Donning PPE:
·Put on special work clothes and work shoes
• Wash hands~ Put on disposable surgical cal)~ Put on medical protective mask (N95)
• Put on inner disposable nitrite/latex gloves
• Put on goggles and protective clothing (note: if wearing protective clothing without foot covers, please also put on separate waterproof boot covers), put on a disposable isolation gown (if required in the specific work zone) and face shield/powered air-purifying respirator(if required in the specific work zone)
• flut on outer disposable latex gloves.
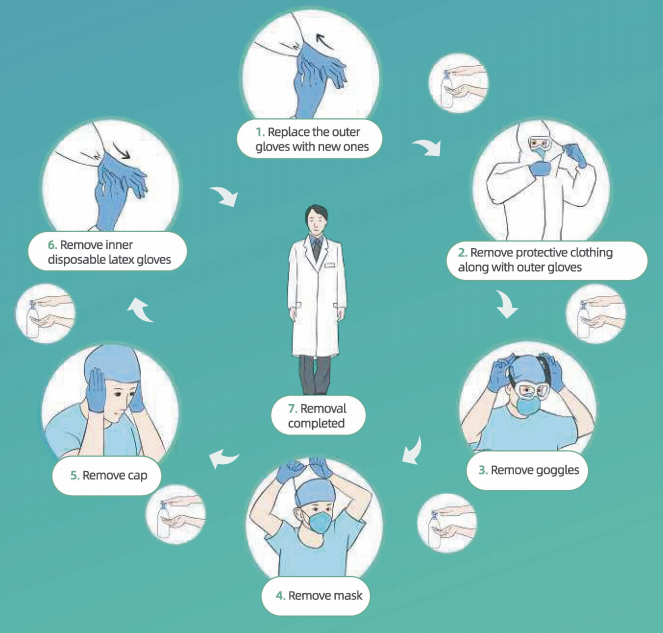
Protocol for Removing PPE:
·Wash hands and remove visible bodily fluids/blood contaminants on the outer surfaces at both hands
• Wash hands replace outer gloves with new gloves
• Remove powered air-purifying respirator m self-priming filter-type full-face mask/mask (if used)
• Wash hands
• Remove disposable gowns along with outer gloves (if used)
• Wash hands and put on outer gloves
• Enter Removal Area
No. ① • Wash hands and remove protective clothing along with outer gloves (for gloves and protective clothing, turn inside out, while rolling them down) (note: if used, remove the waterproof boot covers with clothing) • Wash hands • Enter Removal Area
No. ②• Wash hands and remove goggles • Wash hands and remove mask • Wash hands and remove cap • Wash hands and remove innei disposable latex gloves • Wash hands and leave Removal Area
No.③ • Wash hands, take a shower, put on clean clothes and enter the clean area.
2. Disinfection Procedures for COVID-19 Isolation Ward Area
2.1 Disinfection for Floor and Walls
(1) Visible pollutants shall be completely removed before disinfection and handled in accordance with disposal procedures of blood and bodily fluid spills;
(2) Disinfect the floor and walls with 1000 mg/L chlorine-containing disinfectant through floor mopping, spraying or wiping;
(3) Make sure that disinfection is conducted for at least 30 minutes;
(4) Carry out disinfection three times a day and repeat the procedure at any time when there is contamination.
2.2 Disinfection of Object Surfaces
(1) Visible pollutants should be completely removed before disinfection and handled in accordance with disposal procedures of blood and bodily fluid spills;
(2) Wipe the surfaces of objects with 1000 mg/L chlorine-containing disinfectant or wipes with effective chlorine; wait for 30 minutes and then rinse with clean water. Perform disinfection procedure three times a day (repeat at any time when contamination is suspected);
(3) Wipe cleaner regions first, then more contaminated regions: first wipe the object surfaces that are not frequently touched, and then wipe the object surfaces that are frequently touched. (Once an object surface is wiped clean, replace the used wipe with a new one).
2.3 Air Disinfection
(1) Plasma air sterilizers can be used and continuously run for air disinfection in an environment with human activity;
(2) If there is no plasma air sterilizers, use ultraviolet lamps for 1 hour each time. Perform this operation three times a day.
2.4 Disposal of Fecal Matter and Sewage
(1) Before being discharged into the municipal drainage system, fecal matter and sewage must be disinfected by treating with chlorine-containing disinfectant (for the initial treatment, the active chlorine must be more than 40 mg/L). Make sure the disinfection time is at least 1.5 hours;
(2) The concentration of total residual chlorine in the disinfected sewage should reach 1 O mg/L.
3. Disposal Procedures for Spills of COVID-19 Patient Blood/Fluids
3.1 For spills of a small volume(< 10 ml) of blood/bodily fluids:
(1) Option 1: The spills should be covered with chlorine-containing disinfecting wipes (containing 5000 mg/L effective chlorine) and carefully removed, then the surfaces of the object should be wiped twice with chlorine-containing disinfecting wipes (containing 500 mg/L effective chlorine);
(2) Option 2: Carefully remove the spills with disposable absorbent materials such as gauze, wipes, etc., which have been soaked in 5000 mg/L chlorine-containing disinfecting solution.
3.2 For spills of a large volume(> 1 O ml) of blood and bodily fluids:
(1) First, place signs to indicate the presence of a spill;
(2) Perform disposal procedures according to Option 1 or 2 described below:
① Option 1: Absorb the spilled fluids for 30 minutes with a clean absorbent towel (containing peroxyacetic acid that can absorb up to 1 L of liquid per towel) and then clean the contaminated area after removing the pollutants.
②Option 2: Completely cover the spill with disinfectant powder or bleach powder containing water-absorbing ingredients or completely cover it with disposable water-absorbing materials and then pour a sufficient amount of 10,000 mg/L chlorine-containing disinfectant onto the water-absorbing material (or cover with a dry towel which will be subjected to high-level disinfection). Leave for at least 30 minutes before carefully removing the spill.
(3) Fecal matter, secretions, vomit, etc. from patients shall be collected into special containers and disinfected for 2 hours by a 20,000 mg/L chlorine-containing disinfectant at a spill-to-disinfectant ratio of 1 :2.
(4) After removing the spills, disinfect the surfaces of the polluted environment or objects.
(5) The containers that hold the contaminants can be soaked and disinfected with 5,000 mg/L active chlorine-containing disinfectant for 30 minutes and then cleaned.
(6) The collected pollutants should be disposed of as medical waste.
(7) The used items should be put into double-layer medical waste bags and disposed of as medical waste.
4. Disinfection of COVID-19 Related Reusable Medical Devices
4.1 Disinfection of powered air-purifying respirator
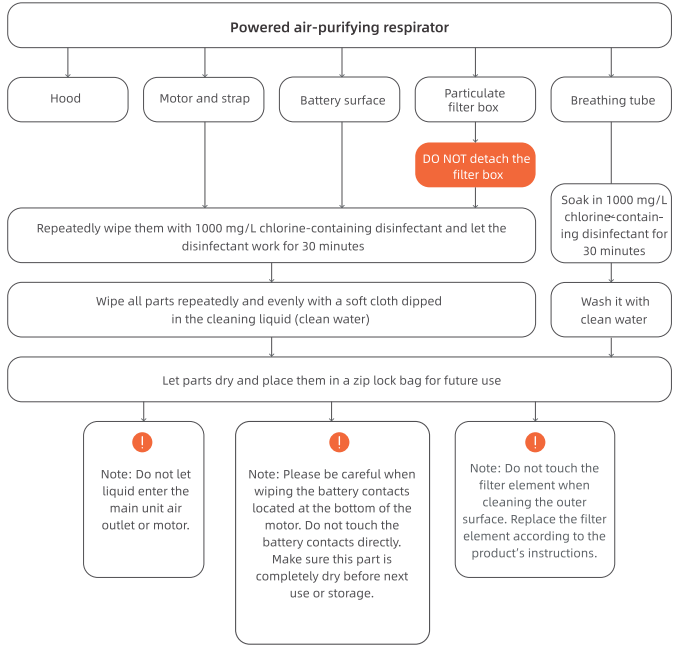
Note: The disinfection procedures for protective hood described above are only for reusable protective hoods (excluding disposable protective hoods).
4.2 Cleaning and Disinfection Procedures for Digestive Endoscopy and Bronchofibroscopy
(1) Soak the endoscope and reusable valves in 0.23% peroxyacetic acid (confirm the concentration of the disinfectant before use to make sure it will be effective);
(2) Connect the perfusion line of each channel of the endoscope, inject 0.23% peroxyacetic acid liquid into the line with a 50 ml syringe until fully filled, and wait for 5 minutes;
(3) Detach the perfusion line and wash each cavity and valve of the endoscope with a disposable special cleaning brush;
(4) Put the valves into an ultrasonic oscillator containing enzyme to oscillate it. Connect the perfusion line of each channel with the endoscope. Inject 0.23% peroxyacetic acid into the line with a 50 ml syringe and flush the line continuously for 5 minutes. Inject air to dry it for 1 minute;
(5) Inject clean water into the line with a 50 ml syringe and flush the line continuously for 3 minutes. Inject air to dry it for 1 minute;
(6) Perform a leakage test on the endoscope;
(7) Put in an automatic endoscopic washing and disinfection machine. Set a high level of disinfection for treatment;
(8) Send the devices to the disinfection supply center to undergo sterilization with ethylene oxide.
4.3 Pre-treatment of Other Reusable Medical Devices
(1) If there are no visible pollutants, soak the device in 1 ooo mg/l chlorine-containing disinfectant for at least 30 minutes;
(2) If there are any visible pollutants, soak the device in 5000 mg/l chlorine-containing disinfectant for at least 30 minutes;
(3) After drying, pack and fully enclose the devices and send them to the disinfection supply center.
5.Disinfection Procedures for Infectious Fabrics of Suspected or Confirmed Patients
5.1 Infectious fabrics
(1) Clothes, bed sheets, bed covers and pillowcases used by patients;
(2) Ward area bed curtains;
(3) Floor towels used for environmental cleaning.
5.2 Collection methods
(1) First, pack the fabrics into a disposable water-soluble plastic bag and seal the bag with matching cable ties;
(2) Then, pack this bag into another plastic bag, seal the bag with cable ties in a goose neck fashion;
(3) Finally, pack the plastic bag into a yellow fabric bag and seal the bag with cable ties;
(4) Attach a special infection label and the department name. Send the bag to the laundry room.
5.3 Storage and washing
(1) Infectious fabrics should be separated from other infectious fabrics (non-COVID-19) and washed in a dedicated washing machine;
(2) Wash and disinfect these fabrics with chlorine-containing disinfectant at 90 °c for at least 30 minutes.
5.4 Disinfection of transport tools
(1) Special transport tools should be used specifically for transporting infectious fabrics;
(2) The tools shall be disinfected immediately each time after being used for transporting infectious fabrics;
(3) The transport tools should be wiped with chlorine-containing disinfectant (with 1000 mg/L active chlorine). Leave disinfectant for 30 minutes before wiping the tools clean with clean water.
6.Disposal Procedures for COVID-19 Related Medical Waste
(1)All waste generated from suspected or confirmed patients shall be disposed of as medical waste;
(2) Put the medical waste into a double-layer medical waste bag, seal the bag with cable ties in a gooseneck fashion and spray the bag with 1000 mg/L chlorinecontaining disinfectant;
(3) Put sharp objects into a special plastic box, seal the box and spray the box with 1 ooo mg/L chlorine-containing disinfectant;
(4) Put the bagged waste into a medical waste transfer box, attach a special infection label, fully enclose the box and transfer it;
(5) Transfer the waste to a temporary storage point for medical waste along a specified route at a fixed time point and store the waste separately at a fixed location;
(6) The medical waste shall be collected and disposed of by an approved medical waste disposal provider.
7.Procedures for Taking Remedial Actions against Occupational Exposure to COVID-19
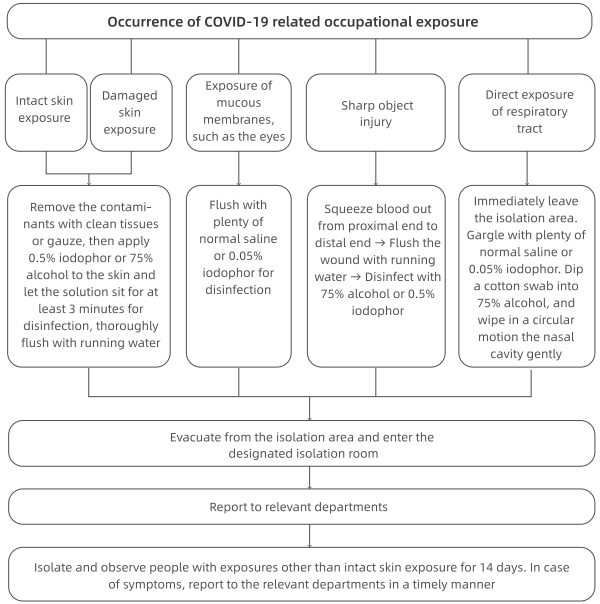
(1) Skin exposure: The skin is directly contaminated by a large amount of visible bodily fluids, blood, secretions or fecal matter from the patient.
(2) Mucous membrane exposure: Mucous membranes, such as the eyes and respiratory tract are directly contaminated by visible bodily fluids, blood, secretions or fecal matter from the patient.
(3) Sharp object injury: Piercing of the body by sharp objects that were directly exposed to the patient's bodily fluids, blood, secretions or fecal matter.
(4) Direct exposure of respiratory tract: Falling off of a mask, exposing the mouth or nose to a confirmed patient (1 miter away) who is not wearing a mask.
8.Surgical Operations for Suspected or Confirmed Patients
8.1 Requirements for Operation Rooms and Staff PPE
(1) Arrange the patient in a negative pressure operating room. Verify the temperature, humidity and air pressure in the operation room;
(2) Prepare all required items for the operation and use disposable surgical items if possible;
(3) All surgical personnel (including surgeons, anesthesiologists, hand-washing nurses, and charge nurses in operating room) should put on their PPE in the buffer room before entering the operating room: Put on double caps, medical protective mask (N95), medical goggles, medical protective clothing, boot covers, latex gloves, and powered air-purifying respirator;
(4) The surgeons and the hand-washing nurses should wear disposable sterile operating clothes and sterile gloves in addition to the PPE as mentioned above;
(5) Patients should wear disposable caps and disposable surgical masks according to their situation;
(6) The charge nurses in the buffer room are responsible for delivering items from the buffer area to the negative pressure operating room;
(7) During the operation, the buffer room and the operating room shall be tightly closed, and the operation must be carried out only if the operation room is under negative pressure;
(8) Irrelevant personnel shall be excluded from entering the operating room.
8.2 Procedures for Final Disinfection
(1) Medical waste shall be disposed of as COVID-19 related medical waste;
(2) Reusable medical devices shall be disinfected according to the disinfection procedures of SARS-CoV-2 related reusable medical devices;
(3) Medical fabrics shall be disinfected and disposed of according to the disinfection procedures for SARS-CoV-2 related infectious fabrics;
(4) Surfaces of objects (instruments and devices including device table, operating table, operating bed, etc.);
① Visible blood/bodily fluid pollutants shall be completely removed before disinfection (handled in accordance with disposal procedures of blood and bodily fluid spills). ② All surfaces shall be wiped with a disinfectant containing 1000 mg/L active chlorine and allowed to sit for 30 minutes with the disinfectant.
(5) Floors and walls:
① Visible blood/bodily fluid pollutants shall be completely removed before disinfection (handled in accordance with disposal procedures of blood and bodily fluid spills).
② All surfaces shall be wiped with a disinfectant containing 1000 mg/L active chlorine and allowed to sit for 30 minutes with the disinfectant.
(6) Indoor air: Turn off the fan filter unit (FFU). Disinfect the air by irradiation by ultraviolet lamp for at least 1 hour. Turn on the FFU to purify the air automatically for at least 2 hours.
9. Procedures for Handling Bodies of Deceased Suspected or Confirmed Patients
(1) Staff PPE: The staff must make sure they are fully protected by wearing work clothes, disposable surgical caps, disposable gloves and thick rubber gloves with long sleeves, medical disposable protective clothing, medical protective masks (N95) or powered air purifying respirators (PAPRs), protective face shields, work shoes or rubber boots, waterproof boot covers, waterproof aprons or waterproof isolation gowns, etc.
(2) Corpse care: Fill all openings or wounds the patient may have, such as mouth, nose, ears, anus and tracheotomy openings, by using cotton balls or gauze dipped in 3000-5000 mg/L chlorine-containing disinfectant or 0.5% peroxyacetic acid.
(3) Wrapping: Wrap the corpse with a double-layer cloth sheet soaked with disinfectant, and pack it into a double-layer, sealed, leak-proof corpse wrapping sheet soaked with chlorine containing disinfectant.
(4) The body shall be transferred by the staff in the isolation ward of the hospital via the contaminated area to the special elevator, out of the ward and then directly transported to a specified location for cremation by a special vehicle as soon as possible.
(5) Final disinfection: Perform final disinfection of the ward and the elevator.
V. Digital Support for Epidemic Prevention and Control
1. Reduce the Risk of Cross Infection when Patients Seek Medical Care
(1) Guide the public to get access to non-emergency services such as chronic diseases treatment on line so as to decrease the number of visitors in healthcare facilities. Doing so minimizes the risk of cross infection.
(2) Patients who must visit healthcare facilities should make an appointment through other means, including an Internet portal, which provides necessary guidance in transportation, parking, arrival time, protective measures, triage information, indoor navigation, etc. Collect comprehensive information online by patients in advance to improve the efficiency of diagnosis and treatment and limit the duration of the patient's visit.
(3) Encourage patients to take full advantage of digital self-service devices to avoid contact with others so as to lower the risk of cross infections.
2.Lower Work Intensity and Infection Risk of Medical Personnel
(1) Collect shared knowledge and experience of experts through remote consultation and multidiscipline team (MDT) to offer the optimum therapeutics for difficult and complicated cases.
(2) Take mobile and remote rounds to lower unnecessary exposure risks and work intensity of medical personnel while saving protective supplies.
(3) Access the patients' latest health conditions electronically through health QR codes (note: everyone is required to obtain a GREEN code through the health QR system to travel around the city) and online epidemiological questionnaires in advance to provide triage guidance to the patients, especially those with fever or suspected cases, while effectively preventing the risk of infection.
(4) Electronic health records of patients in fever clinics and the CT imaging Al system for C0VID-19 can help reduce the work intensity, quickly identify highly-suspected cases and avoid missed diagnoses.
3.Rapid Response to Emergency Needs of COVID-19 Containment
(1) Basic digital resources required by a cloud-based hospital system allows for immediate usage of the information systems needed for emergency response to the epidemic, such as the digital systems equipped for newly established fever clinics, fever observation rooms and isolation wards.
(2) Utilize the hospital information system based on the Internet infrastructure frame to conduct online training for healthcare workers and one-click deployment system, and to facilitate the operation and support engineers to perform remote maintenance and new functions update for medical care.
[ FAHZU lnternert + Hospital - A Model for Online Healthcare ]
Since the outbreak of COVID 19, FAHZU Internet+ Hospital quickly shifted to offer online healthcare through Zhejiang's Online Medical Platform with 24-hour free on line consultation, providing telemedicine service to patients in China and even around the world. Patients are provided access to the first-rate medical services of FAHZU at home, which reduces the chances of transmission and cross infection as a result of their visits to the hospital. As of March 14, over 10,000 people have used the FAHZU Internet+ Hospital online service.
• Instructions for Zhejiang Online Medical Platform:
① Download Alipay app;
② Open Alipay (China Version) and find "Zhejiang Provincial Online Medical Platform" ;
③ Choose a hospital (The First Affiliated Hospital, Zhejiang University School of Medicine);
④ Post your question and wait for a doctor to respond;
⑤ A notification will pop up when a doctor replies. Then open Ali pay and click Friends;
⑥ Click Zhejiang Online Medical Platform to see more details and start your consultation.
[ Establishing the International Medical Expert Communication Platform of the First Affiliated Hospital, Zhejiang University School of Medicine l Due to the spread of the COVID-19 epidemic, the First Affiliated Hospital, Zhejiang University School of Medicine (FAHZU) and Alibaba jointly established the International Medical Expert Communication Platform of FAHZU with an aim to improve the quality of care and treatment and promote the sharing of global information resource. The platform allows medical experts all over the world to connect and share their invaluable experience in the fight against COVID-19 through instant messaging with real-time translation, remote video conferencing, etc.
• Instructions on the International Medical Expert Communication Platform of The First Affiliated Hospital, Zhejiang University School of Medicine
① Visit www.dingtalk.com/en to download DingTalk app.
② Sign up with your personal information (Name and Phone Number) and log in.
③ Apply to join the International Medical Expert Communication Platform of FAHZU: Method 1: Join by team code. Select "Contacts" > "Join Team" > "Join by Team Code" , then enter the Input ID: 'YQDKl 170'. Method 2: Join by scanning the QR code of the International Medical Expert Communication Platform of FAHZU.
④ Fill out your information to join. Enter your name, country and medical institution.
⑤ Join the FAHZU group chat after the ad min has approved.
⑥ After joining the group chat, medical staff can send instant messages assisted by Al translation, receive remote video guidance, and access to medical treatment guidelines.

I.Personalized, Collaborative and Multidisciplinary Management
FAHZU is a designated hospital for COVID-19 patients, especially severe and critically ill individuals whose condition changes rapidly, often with multiple organs infected and requiring the support from the multidisciplinary team (MDT). Since the outbreak, FAHZU established an expert team composed of doctors from the Departments of Infectious Diseases, Respiratory Medicine, ICU, Laboratory Medicine, Radiology, Ultrasound, Pharmacy, Traditional Chinese Medicine, Psychology, Respiratory Therapy, Rehabilitation, Nutrition, Nursing, etc. A comprehensive multidisciplinary diagnosis and treatment mechanism has been established in which doctors both inside and outside the isolation wards can discuss patients'conditions every day via video conference. This allows for them to determine scientific, integrated and customized treatment strategies for every severe and critically ill patient.
Sound decision-making is the key to MDT discussion. During the discussion, experts from different departments focus on issues from their specialized fields as well as critical issues to diagnoses and treatment. The final treatment solution is determined by experienced experts through various discussions of different opinions and advice.
Systematic analysis is at the core of MDT discussion. Elderly patients with underlying health conditions are prone to becoming critically ill. While closely monitoring the progression of COVID-19, the patient's basic status, complications and daily examination results should be analyzed comprehensively to see how the disease will progress. It is necessary to intervene in advance to stop the disease from deteriorating and to take proactive measures such as antivirals, oxygen therapy, and nutritional support.
The goal of MDT discussion is to achieve personalized treatment. The treatment plan should be adjusted to each person when considering the differences among individuals, courses of disease, and patient types.
Our experience is that MDT collaboration can greatly improve the effectiveness of the diagnosis and treatment of COVID-19.
II.Etiology and Inflammation Indicators
1.Detection of SARS-CoV-2 Nucleic Acid
1.1 Specimen Collection Appropriate specimens, collection methodds and collection timing are important to improve detection sensitivity. Specimen types include: upper airway specimens (pharyngeal swabs, nasal swabs, nasopharyngeal secretions), lower airway specimens (sputum, airway secretions, bronchoalveolar lavage fluid), blood, feces, urine and conjunctiva[ secretions. Sputum and other lower respiratory tract specimens have a high positive rate of nucleic acids and should be collected preferentially. SARS-CoV-2 preferentially proliferates in type II alveolar cells (AT2) and peak of viral shedding appears 3 to 5 days after the onset of disease. Therefore, if the nucleic acid test is negative at the beginning, samples should continue to be collected and tested on subsequent days.
1.2 Nucleic Acid Detection Nucleic acid testing is the preferred method for diagnosing SARS-CoV-2 infection. The testing process according to the kit instructions is as follows: Specimens are pre-processed, and the virus is lysed to extract nucleic acids. The three specific genes of SARS-CoV-2, namely the Open Reading Frame la/b (ORFla/b), nucleocapsid protein (N), and envelope protein (E) genes, are then amplified by real-time quantitative PCR technology. The amplified genes are detected by fluorescence intensity. Criteria of positive nucleic acid results are: ORFla/b gene is positive, and/or N gene/E gene are positive. The combined detection of nucleic acids from multiple types of specimens can improve the diagnostic accuracy. Among patients with confirmed positive nucleic acid in respiratory tract, about 30% - 40% of these patients have detected viral nucleic acid in the blood and about 50% - 60% of patients have detected viral nucleic acid in feces. However, the positive rate of nucleic acid testing in urine samples is quite low. Combined testing with specimens from respiratory tract, feces, blood and other types of specimens is helpful for improving the diagnostic sensitivity of suspected cases, monitoring treatment efficacy and the management of post-discharge isolation measures.
2. Virus Isolation and Culture Virus culture must be performed in a laboratory with qualified Biosafety Level 3 (BSL-3). The process is briefly described as follows: Fresh samples of the patient's sputum, feces, etc. are obtained and inoculated on Vero-E6 cells for virus culture. The cytopathic effect (CPE) is observed after 96 hours. Detection of viral nucleic acid in the culture medium indicates a successful culture. Virus titer measurement: After diluting the virus stock concentration by a factor of 10 in series, the TCIDS0 is determined by the micro-cytopathic method. Otherwise, viral viability is determined by plaque forming unit (PFU).
3. Detection of Serum Antibody
Specific antibodies are produced after SARS-CoV-2 infection. Serum antibody determination methods include colloidal gold immunochromatography, ELISA, chemiluminescence immunoassay, etc. Positive serum-specific lgM, or specific lgG antibody titer in the recovery phase ~4 times higher than that in the acute phase, can be used as diagnostic criteria for suspected patients with negative nucleic acid detection. During follow-up monitoring, lgM is detectable 10 days after symptom onset and lgG is detectable 12 days after symptom onset. The viral load gradually decreases with the increase of serum antibody levels.
4.Detecting Indicators of Inflammatory Response It is recommended to conduct tests of (-reactive protein, procalcitonin, ferritin, •-dimer, total and subpopulations of lymphocytes, IL-4, IL-6, IL-10, TNF-a, INF-y and other indicators of inflammation and immune status, which can help evaluate clinical progress, alert severe and critical tendencies, and provide a basis for the formulation of treatment strategies. Most patients with C0VID-19 have a normal level of procalcitonin with significantly increased levels of (-reactive protein. A rapid and significantly elevated (-reactive protein level indicates a possibility of secondary infection. •-dimer levels are significantly elevated in severe cases, which is a potential risk factor for poor prognosis. Patients with a low total number of lymphocytes at the beginning of the disease generally have a poor prognosis. Severe patients have a progressively decreased number of peripheral blood lymphocytes. The expression levels of IL-6 and IL-10 in severe patients are increased greatly. Monitoring the levels of IL-6 and IL-10 is helpful to assess the risk of progression to a severe condition.
5. Detection of Secondary Bacterial or Fungal Infections Severe and critically ill patients are vulnerable to secondary bacterial or fungal infections. Qualified specimens should be collected from the infection site for bacterial or fungal culture. If secondary lung infection is suspected, sputum coughed from deep in the lungs, tracheal aspirates, bronchoalveolar lavage fluid, and brush specimens should be collected for culture. Timely blood culture should be performed in patients with high fever. Blood cultures drawn from peripheral venous or catheters should be performed in patients with suspected sepsis who had an indwelling catheter. It is recommended that they take blood G test and GM test at least twice a week in addition to fungal culture.
6. Laboratory Safety Biosafety protective measures should be determined based on different risk levels of experimental process. Personal protection should be taken in accordance with BSL-3 laboratory protection requirements for respiratory tract specimen collection, nucleic acid detection and virus culture operations. Personal protection in accordance with BSL-2 laboratory protection requirement should be carried out for biochemical, immunological tests and other routine laboratory tests. Specimens should be transported in special transport tanks and boxes that meet biosafety requirements. All laboratory waste should be strictly autoclaved.
Ill. Imaging Findings of COVID-19 Patients
Thoracic imaging is of great value in the diagnosis of C0VID-19, monitoring of therapeutic efficacy, and patient discharge assessment. A high-resolution CT is highly preferable. Portable chest X-rays are helpful for critically ill patients who are immobile. CT for baseline evaluation of patients with C0VID-19 is usually performed on the day of admission, or if ideal therapeutic efficacy is not reached, it can be re-performed after 2 to 3 days. If symptoms are stable or improved after treatment, the chest CT scan can be reviewed after 5 to 7 days. Daily routine portable chest X-rays are recommended for critically ill patients.
C0VID-19 at the early stage often presents with multifocal patchy shadows or ground glass opacities located in the lung periphery, subpleural area, and both lower lobes on chest CT scans. The long axis of the lesion is mostly parallel to the pleura. Interlobular septa I thickening and intralobular interstitial thickening, displaying as subpleural reticulation namely a "crazy paving" pattern, is observed in some ground glass opacities. A small number of cases may show solitary, local lesions, or nodular/ patchy lesion distributed consistent with bronchus with peripheral ground glass opacities changes. Disease progression mostly occurs in the course of 7-10 days, with enlarged and increased density of the lesions compared with previous images, and consolidated lesions with air bronchogram sign. Critical cases may show further expanded consolidation, with the whole lung density showing increased opacity, sometimes known as a "white lung". After the condition is relieved, the ground glass opacities can be completely absorbed, and some consolidation lesions will leave fibrotic stripes or subpleural reticulation. Patients with multiple lobular involvement, especially those with expanded lesions should be observed for disease exacerbation. Those with typical CT pulmonary manifestations should be isolated and undergo continuous nucleic acid tests even if the nucleic acid test of SAR-CoV-2 is negative.
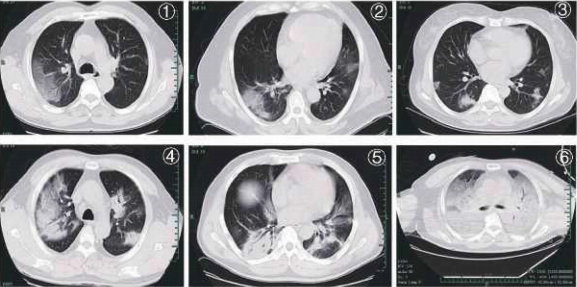
Typical CT features of C0VID-19 :
Figure 1, Figure 2: patchy ground glass opacities;
Figure 3: nodules and patchy exudation;
Figure 4, Figure 5: multifocal consolidation lesions;
Figure 6: diffuse consolidation, "white lung''.
IV. Application of Bronchoscopy in the Diagnosis and Management of COVID-19 Patients
Flexible bronchoscopy is versatile, easy to use, and well tolerated in mechanically ventilated COVID-19 patients. Its applications include:
(1) Collection of respiratory specimens from the lower respiratory tract (i.e. sputum, endotracheal aspirate, bronchoalveolar lavage) for SARS-CoV-2 or other pathogens guides the selection of appropriate antimicrobials, which may lead to clinical benefits. Our experience indicates that lower respiratory specimens are more likely to be positive for SAR-CoV-2 than upper respiratory specimens.
(2) Can be used for localization of the site of bleeding, cessation of hemoptysis, sputum or blood clots removal; if the site of bleeding is identified by bronchoscopy, local injection of cold saline, epinephrine, vasopressin, or fibrin as well as laser treatment can be performed via the bronchoscope.
(3) Assist in the establishment of artificial airways; guide tracheal intubation or percutaneous tracheotomy.
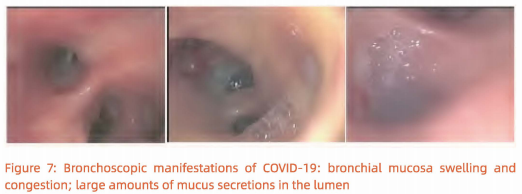
(4) Drugs such as infusion of a-interferon and N-acetylcysteine can be administrated via the bronchoscope. Bronchoscopic views of extensive bronchial mucosa[ hyperemia, swelling, mucus-like secretions in the lumen and jelly-like sputum blocking the airway in critically ill patients. (Figure 7).
V. Diagnosis and Clinical Classification of COVID-19
Early diagnosis, treatment and isolation should be carried out whenever possible. Dynamic monitoring of lung imaging, oxygenation index and cytokine levels are helpful for early identification of patients who may develop into severe and critical cases. A positive result of the nucleic acid of SARS-CoV-2 is the gold standard for the diagnosis of COVID-19. However, considering the possibility of false negatives in nucleic acid detection, suspected cases characteristic manifestations in CT scans can be treated as confirmed cases even if the nucleic acid test is negative. Isolation and continuous tests of multiple specimens should be carried out in such cases.
The diagnostic criteria follow Protocols for the Diagnosis and Treatment of CDVID-2019. A confirmed case is based on epidemiological history (including cluster transmission), clinical manifestations (fever and respiratory symptoms), lung imaging, and results of SARS-CoV-2 nucleic acid detection and serum-specific antibodies.
Clinical Classifications:
1. Mild Cases
The clinical symptoms are mild and no pneumonia manifestations can be found in imaging.
2. Moderate Cases Patients have symptoms such as fever and respiratory tract symptoms, etc. and pneumonia manifestations can be seen in imaging. e Severe Cases Adults who meet any of the following criteria: respiratory rate ≥30 breaths/min; oxygen saturations; 93% at a rest state; arterial partial pressure of oxygen (PaO,)/oxygen concentration (FiO,) ≥300 mm Hg. Patients with> 50% lesions progression within 24 to 48 hours in lung imaging should be treated as severe cases.
4. Critical Cases Meeting any of the following criteria: occurrence of respiratory failure requiring mechanical ventilation; presence of shock; other organ failure that requires monitoring and treatment in the ICU. Critical cases are further divided into early, middle and late stages according to the oxygenation index and compliance of respiratory system.
• Early stage: 100 mmHg < oxygenation index ≤ 150 mmHg ,compliance of respiratory system ≥30mL/cmH,O; without organ failure other than the lungs. The patient has a great chance of recovery through active antiviral, anti-cytokine storm, and supportive treatment.
•Middle stage: 60 mmHg < oxygenation index ≤lOO mmHg; 30 mL/cmH,O > compliance of respiratory system ≥l 5 mL/cmH,O; may be complicated by other mild or moderate dysfunction of other organs.
•Late stage: oxygenation index s≤ 60 mmHg; compliance of respiratory system <15 mL/cmH,O; diffuse consolidation of both lungs that requires the use of ECMO; or failure of other vital organs. The mortality risk is significantly increased.
VI. Antiviral Treatment for Timely Elimination of Pathogens
An early antiviral treatment can reduce the incidence of severe and critical cases. Although there is no clinical evidence for effective antiviral drugs, currently the antiviral strategies based on the characteristics of SAR-CoV-2 are adopted according to Protocols for Diagnosis and Treatment of COVID-19: Prevention, Control, Diagnosis and Management.
1. Antiviral Treatment
At FAHZU, lopinavir/ritonavir (2 capsules, po q12h} combined with arbidol (200 mg po ql 2h) were applied as the basic regimen. From the treatment experience of 49 patients in our hospital, the average time to achieve negative viral nucleic acid test for the first time was 12 days (95% Cl: 8-15 days). The duration of negative nucleic acid test result (negative for more than 2 times consecutively with interval≥24h} was 13.5 days (95% Cl: 9.5 - 17.5 days).
If the basic regimen is not effective, chloroquine phosphate can be used on adults between 18-65 years old (weight
≤so kg: 500 mg bid; weight≤50 kg: 500 mg bid for first two days, 500 mg qd for following five days).
Interferon nebulization is recommended in Protocols for Diagnosis and Treatment of COVID-19. We recommend that it should be performed in negative-pressure wards rather than general wards due to the possibility of aerosol transmission.
Darunavir/cobicistat has some degree of antiviral activity in viral suppression test in vitro, based on the treatment experience of AIDS patients, and the adverse events are relatively mild. For patients who are intolerant to lopinavir/ritonavir, darunavir/ cobicistat (1 tablet qd) or favipiravir (starting dose of 1600 mg followed by 600 mg tid) is an alternative option after the ethical review. Simultaneous use of three or more antiviral drugs is not recommended.
2. Course of Treatment
The treatment course of chloroquine phosphate should be no more than 7 days. The treatment course of other regimens has not been determined and are usually around 2 weeks. Antiviral drugs should be stopped if nucleic acid test results from sputum specimens remain negative for more than 3 times.
VII. Anti-shock and Anti-hypoxemia Treatment
During the progression from the severe to critically ill stage, patients may develop severe hypoxemia, cytokine cascade and severe infections that might develop into shock, tissue perfusion disorders, and even multiple organ failure. Treatment is aimed at incentive removal and fluid recovery. The artificial liver support system (ALSS} and blood purification can effectively diminish inflammatory mediators and cytokine cascade and prevent the incidence of shock, hypoxemia and respiratory distress syndrome.
1. Usage of Glucocorticoids when Necessary
Appropriate and short-term use of corticosteroids to inhibit cytokine cascade and to prevent disease progression should be considered for patients with severe COVID-19 pneumonia as early as possible. However, a high dose of glucocorticoids should be avoided due to adverse events and complications.
1.1 Indication for Corticosteroids
① for those in severe and critically ill stage
② for those with persistent high fever (temperature above 39°C};
③ for those whose computerized tomography (CT) demonstrated patchy ground-glass attenuation or more than 30% area of the lungs are involved;
④ for those whose CT demonstrated rapid progression (more than 50% area involved in pulmonary CT images within 48 hours);
⑤ for those whose IL-6 is above≥5 ULN.
1.2 Application of Corticosteroids
Initial routine methylprednisolone at a dose of 0.75-1.5 mg/kg intravenously once a day (nearly 40 mg once or twice a day) is recommended. However, methylprednisolone at a dose of 40 mg ql2h can be considered for patients with falling body temperature or for patients with significantly increased cytokines under routine doses of steroid. Even methylprednisolone at a dose of 40 mg-80 mg ql2h can be considered for critical cases. Closely monitor body temperature, blood oxygen saturation, blood routine, c-reactive protein, cytokines, biochemical profile and lung CT every 2 to 3 days during the treatment as necessary. The dosage of methylprednisolone should be halved every 3 to 5 days if medical conditions of patients are improved, the body temperature normalizes, or involved lesions on CT are significantly absorbed. Oral methylprednisolone (Medrol) once a day is recommended when the intravenous dose is reduced to 20 mg per day. The course of corticosteroids in not defined; some experts have suggesting ceasing corticosteroids treatment when patients are nearly recovered.
1.3 Special Consideration during Treatment
① screening of TB by T-SPOT assay, HBV and HCV by antibody assay should be performed before corticosteroid therapy;
② al proton pump inhibitors could be considered to prevent complications;
③ blood glucose should be monitored. High blood glucose should be treated with insulin when necessary;
④ low serum potassium should be corrected;
⑤ liver function should be monitored closely;
⑥ traditional Chinese herbal medicine may be considered for patients who are sweating;
⑦ sedative-hypnotics can be administered temporarily for patients with sleep disorder.
2.Artificial Liver Treatment for Suppression of Cytokine Cascade
The artificial liver support system (ALSS) can conduct plasma exchange, adsorption, perfusion, and filtration of inflammatory mediators such as endotoxins and harmful metabolic substances of small or medium molecular weight. It can also provide serum albumin, coagulation factors, balance fluid volume, electrolytes and acid-base ratio, and manifest anti-cytokine storms, shock, lung inflammation, et al. In doing so it can also help to improve multiple organ functions including the liver and kidney. Thus, it can increase treatment success and reduce the mortality of severe patients.
2.1 Indication for ALSS
① serum inflammatory indicator (such as IL-6) level rises to≥ 5 ULN, or rising rate is ≥el time per day;
② involved area of pulmonary CT or X-ray images ≥10% progression per day;
③ artificial liver support system is required for the treatment of underlying diseases. Patients meeting G) + al, or patients meeting①+②,or patients meeting ③.
2.2 Contraindications There is no absolute contraindication in the treatment of critically ill patients. However, ALSS should be avoided in the following situations:
① Severe bleeding disease or disseminated intravascular coagulation;
② Those who are highly allergic to blood components or drugs used in the treatment process such as plasma, heparin and protamine;
③ Acute cerebrovascular diseases or severe head injury;
④ Chronic cardiac failure, cardiac functional classification;;, grade Ill;
⑤ Uncontrolled hypotension and shock;
⑥ Severe arrhythmia.
Please exchange combined with plasma adsorption or dual plasma molecular adsorption, perfusion, and filtration is recommended according to the patients' situation. 2000 ml of plasma should be exchanged when ALSS is performed. Detailed operating procedures can be found in the Expert Consensus on the Application of Artificial Liver Blood Purification System in the Treatment of Severe and Critical Novel Coronavirus Pneumonia. ALSS significantly reduces the time that critically ill patients stay in the ICU in our hospital. Typically, the levels of serum cytokines such as IL-2/IL-4/IL-6/TNF-a are remarkably decreased, and oxygen saturation is significantly improved after ALSS.
3. Oxygen Therapy for Hypoxemia
Hypoxemia can present due to impaired respiratory functions by COVID-19. Oxygen supplementation treatment can correct hypoxemia, relieving secondary organ damage caused by respiratory distress and hypoxemia.
3.1 Oxygen therapy
(1) Continual oxygen saturation monitoring during oxygen therapy Some patients do not necessarily have impaired oxygenation functions at the onset of infection but may manifest rapid deterioration in oxygenation over time. Therefore, continual monitoring of oxygen saturation is recommended, before and during oxygen therapy.
(2) Oxygen therapy as soon as possible Oxygen therapy is not necessary for patients with oxygen saturation (SpO2) of more than 93% or for patients without obvious symptoms of respiratory distress without oxygen treatment. Oxygen therapy is strongly recommended to the patients with symptoms of respiratory distress. It should be noted that some severe patients with PaO/FiO2 < 300 had no obvious symptoms of respiratory distress. (3) Treatment goal of oxygen therapy The treatment goal of oxygen therapy is to maintain the oxygen saturation {SpO2) at 93%-96% for patients without chronic pulmonary disease and at 88%-92% for patients with chronic type II respiratory failure. Specially, the oxygen concentration should be increased to 92%-95% for patients whose SpO2 drops below 85% frequently during daily activities.
(4) Control oxygen therapy PaO/FiO2 is a sensitive and accurate indicator of oxygenation function. The stability and monitorability of FiO2 are very important for patients with disease progression and PaO/FiO2 below 300 mmHg. Controlled oxygen therapy is the preferred treatment. High-flow nasal cannula (HFNC) oxygen therapy is recommended for patients with the following conditions: SpO2 < 93%; PaO/FiO2 < 300 mmHg (1 mmHg = 0.133 kPa); respiratory rate> 25 times per min at bed; or remarkable progression on X-ray imaging. Patients should wear a surgical mask during HFNC treatment. The airflow of HFNC oxygen therapy should start at a low level and gradually increased up to 40-60 L/min when PaO/FiO2 is between 200-300 mm Hg so that patients do not feel obvious chest tightness and shortness of breath. An initial flow of at least 60 L/min should be given immediately for patients with obvious respiratory distress. Tracheal intubation for patients is dependent on disease progression, systemic status and complication of patients for those with stable situation but with a low oxygenation index ( 60%) HFNC oxygen therapy. Older patients (> 60 years old) with more complications or PaO,/FiO, less than 200 mmHg should be treated in ICU.
3.2 Mechanical Ventilation
(1) Noninvasive Ventilation (NIV) NIV is not strongly recommended in COVID-19 patients who fail HFNC treatment. Some severe patients progress to ARDS rapidly. Excessive inflation pressure may cause gastric distension and intolerance which contribute to aspiration and worsen lung injury. A short-term (less than 2 hours) use of NIV can be closely monitored if the patient has acute left heart failure, chronic obstructive pulmonary disease or is immune compromised. Intubation should be performed as early as possible if improvement of respiratory distress symptoms or PaO/FiO2 is not observed. NIV with a double circuit is recommended. A virus filter should be installed between the mask and the exhalation valve when applying NIV with a single tube. Suitable masks should be chosen to reduce the risk of virus spread through air leakage.
(2) Invasive Mechanical Ventilation
① Principles of invasive mechanical ventilation in critically ill patients It is important to balance the ventilation and oxygenation demands and the risk of mechanical ventilation-related lung injury in the treatment of COVID-19.
• Strictly set the tidal volume to 4 - 8 ml/kg. In general, the lower the lung compliance, the smaller the preset tidal volume should be.
• Maintain the platform pressure< 30 cmH,O (1 cmH,O = 0.098 kPa) and driving pressure < 15 cmH,O.
• Set PEEP according to the ARDS's protocol.
• Ventilation frequency: 18-25 times per minute. Moderate hypercapnia is allowed.
• Administer sedation, analgesia, or muscle relaxant if the tidal volume, platform pressure and driving pressure are too high.
②Lung Recruitment Lung recruitment improves the heterogeneous distribution of lesions in patients with ARDS. However, it may result in severe respiratory and circulatory complications and therefore, the lung recruitment maneuver is not routinely recommended. The assessment of lung expandability should be performed prior to the application.
(3) Prone Position Ventilation Most critically ill patients with COVID-19 respond well to prone ventilation, with a rapid improvement of oxygenation and lung mechanics. Prone ventilation is recommended as a routine strategy for patients with PaO/FiO2 < 150 mmHg or with obvious imaging manifestations without contraindications. Time course recommended for prone ventilation is more than 16 hours each time. The prone ventilation can be ceased once PaO/FiO2 is greater than 150 mm Hg for more than 4 hours in the supine position. Prone ventilation while awake may be attempted for patients who have not been intubated or have no obvious respiratory distress but with impaired oxygenation or have consolidation in gravity-dependent lung zones on lung images. Procedures for at least 4 hours each time is recommended. Prone position can be considered several times per day depending on the effects and tolerance.
(4) Prevention of Regurgitation and Aspiration Gastric residual volume and gastrointestinal function should be routinely evaluated. Appropriate enteral nutrition is recommended to be given as earlier as possible. Nasointestinal feeding and continuous nasogastric decompression are recommended. Enteral nutrition should be suspended and aspiration with so ml syringe be done before transfer. If no contraindication exists, a 30° semi-sitting position is recommended.
(5) Fluid Management Excessive fluid burden worsens hypoxemia in COVID-19 patients. To reduce pulmonary exudation and improve oxygenation, the amount of fluid should be strictly controlled while ensuring the patient's perfusion.
(6) Strategies to Prevent Ventilator-Associated Pneumonia (VAP) VAP bundled strategies should be strictly implemented:
① Select appropriate type of endotracheal tube;
② Use a endotracheal tube with subglottic suction (once every 2 hours, aspirated with 20 ml empty syringe each time);
③ Place the endotracheal tube at the right position and correct depth, fix properly and avoid pulling;
④ Maintain the airbag pressure at 30 - 35 cmH,O (1 cmH,O = 0.098 kPa) and monitor every 4 hours;
⑤ Monitor the airbag pressure and deal with water condensates when the position changes (two people cooperate in dumping and pouring the water condensates into a capped container containing a pre-made disinfectant chlorine solution); deal with secretions accumulated in the airbag;
⑥ Clean up secretions from the mouth and nose timely.
(7) Weaning of Ventilation Sedatives is reduced and discontinued before awakening when the patient's PaO2'FiO2 is more than 150 mmHg. Intubation withdrawal should be performed as earlier as possible if permitted. HFNC or NIV is used for sequential respiratory support after withdrawal.
IV.The Rational Use of Antibiotics to Prevent Secondary Infection
COVID-19 is a disease of viral infection, therefore antibiotics are not recommended to prevent bacterial infection in mild or ordinary patients; it should be used carefully in severe patients based on their conditions. Antibiotics can be used with discretion in patients who have the following conditions: extensive lung lesions; excess bronchial secretions; chronic airway diseases with a history of pathogen colonization in the lower respiratory tract; taking glucocorticoids with a dosage ≥ 20 mg x 7d (in terms of prednisone). The options of antibiotics include quinolones, the second or third generation cephalothins, ~-lactamase inhibitor compounds, etc. The antibiotics should be used for the prevention of bacterial infection in critically severe patients, especially those with invasive mechanical ventilation. The antibiotics such as carbapenems, ~-lactamase inhibitor compounds, linezolid and vancomycin can be used in critically ill patients according to the individual risk factors.
The patient's symptoms, signs and indicators such as blood routine, c-reactive protein, and procalcitonin, need to be closely monitored during the treatment. When the change of a patient's condition is detected, a comprehensive clinical judgment needs to be made. When the secondary infection cannot be ruled out, qualified specimen need to be collected for testing by smear preparation, cultivation, nucleic acid, antigen and antibody, in order to determine the infectious agent as early as possible. Antibiotics can be empirically used in the following conditions
① more expectoration, darker sputum color, especially yellow pus sputum;
② the rise of body temperature which is not due to exacerbation of the original disease;
③ the marked increase of white blood cells and/or neutrophils;
④ procalcitonin≥0.5 ng/mL;
⑤ Exacerbation of oxygenation index or circulatory disturbance that are not caused by the viral infection; and the other conditions suspiciously caused by bacteria infections.
Some COVID-19 patients are at the risk of secondary fungal infections due to weakened cellular immunity caused by viral infections, the use of glucocorticoid and/or broad-spectrum antibiotics. It is necessary to do respiratory secretions microbiological detections such as smear preparation and cultivation for critically ill patients; and provide timely D-Glucose (G-test) and galactomannan (GM-test) of blood or bronchoalveolar lavage fluid for suspected patients.
It is necessary to be vigilant with possible invasive candidiasis infection and anti-fungal therapy. Fluconazole or echinocandin can be used in the following conditions
① patients are given broad-spectrum antibiotics for seven days or more
② patients have parenteral nutrition;
③ patients have invasive examination or treatment;
④ patients have positive candida culture in the specimen obtained from two body parts or more;
⑤ patients have significantly increased results of G-test.
It is necessary to be vigilant with possible invasive pulmonary aspergillosis. Anti-fungal therapy such as voriconazole, posaconazole, or echinocandin are considered to be used in the following conditions
① patients are given glucocorticoid for seven days or more
② patients have agranulocytosis
③ patients have chronic obstructive pulmonary disease and aspergillus culture are tested positive in the specimen obtained from the airway
④ patients have significantly increased results of GM-test.
V. The Balance of Intestinal Microecology and Nutritional Support
Some COVID-19 patients have gastrointestinal symptoms (such as abdominal pain and diarrhea) due to direct viral infection of the intestinal mucosa or antiviral and anti-infective drugs. There has been report that the intestinal microecological balance is broken in COVID-19 patients, manifesting a significant reduction of the intestinal probiotics such as lactobacillus and bifidobacterium. Intestinal microecological imbalance may lead to bacterial translocation and secondary infection, so it is important to maintain the balance of intestinal microecology by microecological modulator and nutritional support.
1. Microecologics Intervention
(1) Microecologics can reduce bacterial translocation and secondary infection. It can increase dominant gut bacteria, inhibit intestinal harmful bacteria, reduce toxin production and reduce infection caused by gut microflora dysbiosis.
(2) Microecologics can improve the gastrointestinal symptoms of patients. It can reduce water in feces, improve fecal character and defecation frequency, and reduce diarrhea by inhibiting intestinal mucosa I atrophy.
(3) The hospital with relevant resources can perform intestinal flora analysis. Therefore, the intestinal flora disturbance can be discovered early according to the results. Antibiotics can be adjusted timely and probiotics can be prescribed. These can reduce the chances of intestinal bacterial translocation and gut-derived infection.
(4) Nutrition support is an important means to maintain intestinal microecological balance.Intestinal nutrition support should be applied timely on the basis of effective evaluations of nutritional risks, gastroenteric functions, and aspiration risks.
1. Nutrition Support
The severe and critically ill COVID-19 patients who are in a state of severe stress are at high nutritional risks. Early evaluations of nutrition risk, gastrointestinal functions and aspiration risks, and timely enteral nutritional support are important to the patient's prognosis.
(1) Oral feeding is preferred. The early intestinal nutrition can provide nutritional support, nourish intestines, improve intestinal mucosa I barrier and intestinal immunity, and maintain intestinal microecology.
(2) Enteral nutrition pathway. Severe and critically ill patients often harbor acute gastrointestinal damages, manifested as abdominal distension, diarrhea, and gastroparesis. For patients with tracheal intubation, intestinal nutrition tube indwelling is recommended for post-pyloric feeding.
(3) Selection of nutrient solution. For patients with intestinal damage, predigested short peptide preparations, which are easy for intestinal absorption and utilization, are recommended. For patients with good intestinal functions, whole-protein preparations with relatively high calories can be selected. For hyperglycemia patients, nutritional preparations which are beneficial to glycemic controlling are recommended.
(4) Energy supply. 25-30 kcal per kg body weight, the target protein content is 1.2-2.0 g/kg daily.
(5) Means of nutritional supply. Pump infusion of nutrients can be used at a uniform speed, starting with a low dosage and gradually increasing. When possible, the nutrients can be heated before feeding to reduce intolerance. (6) The elderly patients who are at high aspiration risks or patients with apparent abdominal distention can be supported by parenteral nutrition temporarily. It can be gradually replaced by independent diet or enteral nutrition after their condition improves.
VI.ECMO Supportfor COVID-19 Patients
COVID-19 is a novel, highly infectious disease primarily targeting pulmonary alveoli, which damages primarily the lungs of critically ill patients and leads to severe respiratory failure. For the application of extracorporeal membrane oxygenation (ECMO) in COVID-19 treatment, medical professionals need to pay close attention to the following: the time and means of intervention, anticoagulant and bleeding, coordination with mechanical ventilation, awake ECMO and the early rehabilitation training, strategy of handling for complications.
1. ECMO Intervention Timing
1.1 Salvage ECMO In the state of mechanical ventilation support, measures such as lung protective ventilation strategy and prone position ventilation have been taken for 72 h. With the onset of one of the following conditions, salvage ECMO intervention needs to be considered.
(1) Pa02/Fi02 < 80 mm Hg (regardless of what the PEEP level is);
(2) Pplat s 30 mm Hg, Pa CO2> 55 mm Hg;
(3) The onset of pneumothorax, air leakage> l /3 tidal volume, duration> 48 h;
(4) Circulation deterioration, the dosage of norepinephrine > 1 µg/(kgxmin);
(5) Cardio-pulmonary resuscitation in vitro life support ECPR.
1.2 Replacement ECMO When the patient is not suitable for long-term mechanical ventilation support, i.e., the patient is not able to obtain the expected results, ECMO replacement needs to be adopted immediately. With the onset of one of the following conditions, ECMO replacement needs to be considered.
(1) Decreased lung compliance. After the pulmonary recruitment maneuver, the compliance of the respiratory system< 10 mL/cmH,O;
(2) Persistent exacerbation of pneumomediastinum or subcutaneous emphysema. And the parameters of mechanical ventilation support cannot be reduced within 48 h, according to the estimation;
(3) Pa02/Fi02 < 100 mmHg. And it cannot be improved by routine methods in 72 h.
1.3 Early Awake ECMO
Early awake ECMO can be applied to patients who have been supported by mechanical ventilation with the expected high parameters for more than 7 days and who meet the necessary conditions of awake ECMO. They might benefit from it. All the following conditions must be met:
(1) The patient is in a clear state of consciousness and is fully compliant. He or she understands how ECMO works and its maintenance requirements;
(2) The patient is not complicated with neuromuscular diseases;
(3) Pulmonary damage score Murry> 2.5
(4) Few pulmonary secretions. The time interval between the two airway suction procedures> 4 h;
(5) Stable hemodynamics. Vasoactive agents are not required for assistance.
2.Cathetering Methods
Because the ECMO supporting time for most COVID-19 patients is greater than 7 days, the seldinger method should be used as much as possible for the ultrasound guided peripheral catheter insertion, which reduces the bleeding damages and infection risks brought about by intravascular cathterization by venous angiotomy, especially for the early awake ECMO patients. lntravascular catheterization by venous angiotomy may be considered only for the patients with bad blood vessel conditions, or the patients whose catheterization cannot be identified and selected by ultrasound, or the patients whose seldinger technique failed.
3. Mode Selection
(1) The first choice for the patients of respiratory impairment is the V-V mode. The V-A mode should not be the first option just because of the possible circulation problems.
(2) For the respiratory failure patients complicated with cardiac impairment, PaO2/FiO2 < 100 mm Hg, the V-A-V mode ought to be selected with the total flux> 6 Umin and V/A = 0.5/0.5 is maintained by current limiting.
(3) For the COVID-19 patients without severe respiratory failure but complicated with serious cardiovascular outcomes leading to cardiogenic shock, the V-A assisted by ECMO mode ought to be selected. But IPPV support is still needed and the awake ECMO should be avoided.
4.Flux Set-value and Target Oxygen Supply
(1) The initial flux> 80% cardiac output (CO) with a self-cycling ratio< 30%.
(2) SPO2 > 90% is to be maintained. FiO2 < 0.5 is supported by mechanical ventilation or the other oxygen therapy.
(3) To ensure the target flux, 22 Fr (24 Fr} vein access canula is the first choice for the patient with a body weight below (above) BO kg.
5. Ventilation Setting Normal ventilation maintenance by adjusting the sweep gas level:
(1) The initial air flow is set to be Flow: sweep gas= 1 :1. The basic target is to maintain Pa CO2< 45mmHg. For the patients complicated with COPD, Pa CO2< 80% basal level.
(2) The patient's
spontaneous respiratory strength and respiratory rate (RR} should be maintained,
with 10
(3) The sweep gas setup of the V-A mode needs to ensure the 7.35-7.45 PH value of the bloodstream out of the oxygenator membrane.
6. Anti-Coagulation and Bleeding Prevention
(1) For the patients without active bleeding, without visceral bleeding, and with platelet count> 50xl09/L, the recommended initial heparin dosage is 50 U/kg.
(2) For the patients complicated with bleeding or with platelet count < 50xl 09/L, the recommended initial heparin dosage is 25 U/kg.
(3) The activated partial thromboplastin time (aPPT} being 40-60 sec is proposed to be the target of anticoagulation maintenance dosage. The trend of D-dimer change should be considered at the same time.
(4) Heparin-free operation may be performed in the following circumstances: the ECMO support must continue but there is fatal bleeding or active bleeding that has to be controlled; whole heparin coated loop and catheterization with blood flow> 3 L/min. The recommend operation time< 24 hour. Replacement devices and consumables need to be prepared.
(5) Heparin resistance. Under some conditions of heparin usage, a PTT is not able to reach the standard and blood coagulation happens. In this case, the activity of plasma antithrombin Ill (ATIII) needs to be monitored. If the activity reduces, fresh frozen plasma needs to be supplemented to restore heparin sensitivity.
(6) Heparin induced thrombopenia (HIT). When HIT happens, we recommend to perform plasma exchange therapy, or to replace heparin with argatroban.
7. Weaning from ECMO and Mechanical Ventilation
(1) If a patient treated by v-v ECMO combined with mechanical ventilation satisfies the awake ECMO condition, we suggest to first try to remove the artificial airway, unless the patient has ECMO related complications, or the expected time of removal of all the assisting machines is less than 48 h.
(2) For a patient who has too much airway secretions that frequent artificial suction clearance is needed, who is expected to have a long-term mechanical ventilation support, who satisfies the conditions PaO,/FiO, > 150 mm Hg and time> 48 h, whose lung image changes for the better, and whose damages related to mechanical ventilation pressure have been controlled, the ECMO assistance may be removed. It is not recommended to keep ECMO intubation.

XI. Convalescent Plasma Therapy for COVID-19 Patients
Since Behring and Kitasato reported the therapeutic effects of diphtheria antitoxin plasma in 1 B91, plasma therapy has become an important means of pathogen immunotherapy for acute infectious diseases. The disease progression is rapid for severe and critically ill patients of an emerging infectious disease. In the early phase, the pathogens damage the target organs directly and then lead to severe immuno-pathological damage. The passive immune antibodies can effectively and directly neutralize the pathogens, which reduces the damage of the target organs and then block the subsequent immune-pathological damages. During multiple global pandemic outbreaks, WHO also emphasized that "convalescent plasma therapy is one of the most recommended potential therapies, and it has been used during other epidemic outbreaks". Since the outbreak of COVID-19, the initial mortality rate was rather high due to the lack of specific and effective treatments. As mortality rate is an important metric that the public concerns, clinic treatments which can reduce the fatality rate of critical cases effectively are key to avoid public panic. As a provincial-level hospital in Zhejiang province, we have been responsible to treat the patients from Hangzhou and the critically ill patients of the province. There are abundant potential convalescent plasma donors and critically ill patients who need convalescent plasma treatment in our hospital.
1. Plasma collection
In addition to the common requirements of blood donation and procedures, the following details should be noted.
1.1 Donors At least two weeks after recovery and being discharged (the nucleic acid test of the sample taken from the lower respiratory tract remains negative~14 days). 18,≤ Age≥ 55. The body weight>50 kg (for male) or > 45 kg (for female). At least one week since last glucocorticoid usage. More than two weeks since last blood donation.
1.2 Collection Method
Plasmapheresis, 200-400 ml each time (based on medical consultation).
1.3 Post-Collection Testing In addition to the general quality test and the test of blood-borne disease, the blood samples need to be tested for:
(1) Nucleic acid testing for SARS-CoV-2;
(2) 160-fold dilution for the qualitative test of SARS-CoV-2 specific lgG and lgM detection; or 320-fold dilution for the qualitative test of whole antibody detection. If possible, keep> 3 ml plasma for the viral neutralization experiments. The following should be noted. During the comparison of virus neutralization titer and luminescent lgG antibody quantitative detection, we found that the present SARS-CoV-2 specific lgG antibody detection does not fully demonstrate the actual virus neutralization capability of the plasma. Therefore, we suggested the virus neutralization test as the first choice, or test the overall antibody level with the 320-fold dilution of the plasma.
2. Clinical Use of the Convalescent Plasma
2.1 Indication
(1) Severe or critically ill COVID-19 patients tested positive in respiratory tract test;
(2) The COVID-19 patients who are not severe or critically ill, but in a state of immunity suppression; or have low CT values in the virus nucleic acid testing but with a rapid disease progression in the lungs. Note: In principle, the convalescent plasma should not be used on COVID-19 patients with disease course exceeding three weeks. But in clinical applications, we found that the convalescent plasma therapy is effective for patients with a disease course exceeding three weeks and whose virus nucleic acid tests continuously to show positive from respiratory tracts specimen. It can speed up virus clearance, increase the numbers of the plasma lymphocytes and NK cells, reduce the level of plasma lactic acid, and improve renal functions.
2.2 Contraindication
(1) Allergy history of plasma, sodium citrate and methylene blue;
(2) For patients with history of autoimmune system diseases or selective lgA deficiency, the application of convalescent plasma should be evaluated cautiously by clinicians. 2.3 Infusion plan In general, the dosage of convalescent plasma therapy is ~400 ml for one infusion, or~ 200 ml per infusion for multiple infusions.
XII. TCM Classification Therapy to Improve Curative Efficacy
1. Classification and Stage COVID-19 can be divided into early, middle, critical and recovery stages. At the early stage, the disease has two main types: "wet lungs" and "external cold and internal heat." The middle stage is characterized by "intermittent cold and heat." The critical stage is characterized by "internal block of epidemic toxin." The recovery stage is characterized by "qi deficiency in lung-spleen." The disease initially belongs to wet lung syndrome. Due to fever, both intermittent cold and heat treatments are recommended. In the middle stage, cold, dampness, and heat coexist, belonging to "cold-heat mixture" in terms of TCM. Both cold and heat therapy should be considered. According to the theory of TCM, heat should be treated with cold drugs. But cold drugs impair Yang and lead to a cold spleen and stomach and cold-heat mixture in the middle-Jiao. Therefore, in this stage both cold and heat therapies should be considered. Because cold-heat symptoms are commonly seen in COVID-19 patients, the cold-heat therapy is better than other approaches
2. Therapy Based on Classification
(1) Wet lungs Ephedra Herb 6 g, Semen Armeniacae Amarumg 10 g, Coix Seed 30 g, Liquoric Root 6 g, Baical Skullcap Root 15 g, Huoxiang 10 g, Reed Rhizome 30 g, Cyrtomium Rhizome 15 g, Indian Buead 20 g, Chinese Atractylodes Rhizome 12 g, Officinal Magnolia Bark 12 g.
(2) External cold and internal heat Herba Ephedrae 9 g, Raw Gypsum Fibrosum 30 g, Semen Armeniacae Amarumg 10 g, Liquoric Root 6 g, Baical Skullcap Root 15 g, Pericarpium Trichosanthis 20 g, Fructus Aurantii 15 g, Officinal Magnolia Bark 12 g, Tripterospermum Cordifolium 20 g, White Mulberry Root-bark 15 g, Pinellia Tuber 12 g, Indian Buead 20 g, Platycodon Root 9 g.
(3) Intermittent cold-heat Pinellia Tuber 12 g, Baical Skullcap Root 15 g, Golden Thread 6 g, Dried Ginger 6 g, Chinese Date 15 g, Kudzuvine Root 30 g, Costustoot 10 g, Indian Buead 20 g, Thunberg Fritillary Bulb 15 g, Coix Seed 30 g, Liquoric Root 6 g.
(4) Internal block of epidemic toxin Use cheongsimhwan for treatment.
(5) Qi deficiency of lung and spleen Membranous Milkvetch Root 30 g, Pilose Asiabell Root 20 g, Roasted Largehead Atractylodes Rhizome 15 g, Indian Buead 20 g, Fructus Amomi 6 g, Siberian Solomonseal Rhizome 15 g, Pinellia Tuber 10 g, Tangerine Peel 6 g, Wingde Yan Rhizome 20 g, Semen Nelumbinis 15 g, Chinese Date 15 g. Patients in different stages should take different approaches. One dose per day. Boil the medicine in water. Take it every morning and evening.
XIII. Drug Use Management of COVID-19 Patients
COVID-19 patients are often complicated with underlying diseases receiving multiple types of drugs. Therefore, we should pay more attention to the adverse drug reactions and drug interactions so as to avoid drug-induced organ damage and improve the success rate of treatment.
1. Identification of adverse drug reactions
It has been demonstrated that the incidence of abnormal liver function is 51.9% in COVID-19 patients who have received lopinavir/ritonavir combined arbidol antiviral treatment. Multivariate analysis revealed that antiviral agents and more concomitant medications are two independent risk factors of abnormal liver function. Therefore, monitoring of the adverse drug reactions should be strengthened; the unnecessary drug combinations should be reduced. The main adverse reactions of antiviral agents include:
(l) Lopinavir /ritonavir and darunavir/cobicistat: diarrhea, nausea, vomit, the increase of serum aminotransferase, jaundice, dyslipidemia, the increase of lactic acid. Symptoms will recover after drug withdrawal.
(2) Arb idol: the increase of serum aminotransferase and jaundice. When combined with lopinavir, the incidence rate is even higher. The symptoms will recover after drug withdrawal. Sometimes a slowdown of the heart could be induced; thus it is necessary to avoid the combination of arbidol with ~-receptor inhibitors such as metoprolol and propranolol. We suggest to stop taking the drugs when the heart rate drops below 60/min.
(3) Fapilavir: elevation of plasma uric acid, diarrhea, neutropenia, shock, fulminant hepatitis, acute kidney injury. The adverse reactions were commonly seen in elderly patients or patients complicated with cytokine storm.
(4) Chloroquine phosphate: dizziness, headache, nausea, vomit, diarrhea, different kinds of skin rash. The most severe adverse reaction is cardiac arrest. The main adverse reaction is the ocular toxicity. An electrocardiogram needs to be examined before taking the drug. The drug should be prohibited for patients with arrhythmia (e.g., conduction block), retinal disease, or hearing loss.
2. Therapeutic Drug Monitoring
Some antiviral and antibacterial drugs need therapeutic drug monitoring {TDM). Table 1 presents the plasma concentrations of such drugs and their dosage adjustment. Upon the onset of aberrations of plasma drug concentration, the treatment regimens need to be adjusted by considering the clinical symptoms and concomitant drugs.
Table 1 The range of concentrations and points for attention of the common TDM drugs forthe C0VID-19 patients
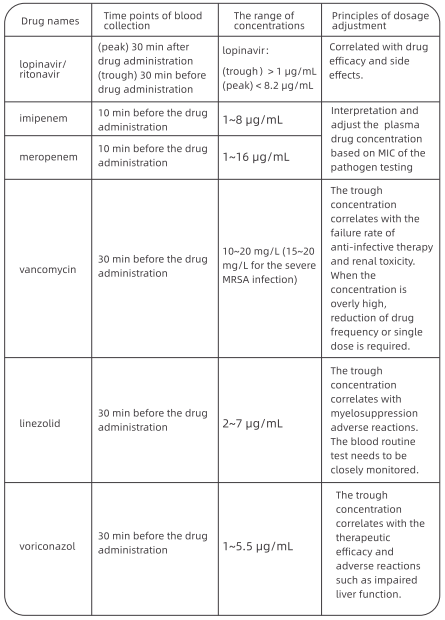
3. Paying attention to the potential drug interactions
Antiviral drugs such as lopinavir/ritonavir are metabolized through the enzyme CYP3A in the liver. When patients receiving concomitant medications, the potential drug interactions need to be carefully screened. Table 2 shows interactions between antiviral drugs and common drugs for underlying diseases.
Table 2 Interactions between antiviral drugs and common drugs for underlying
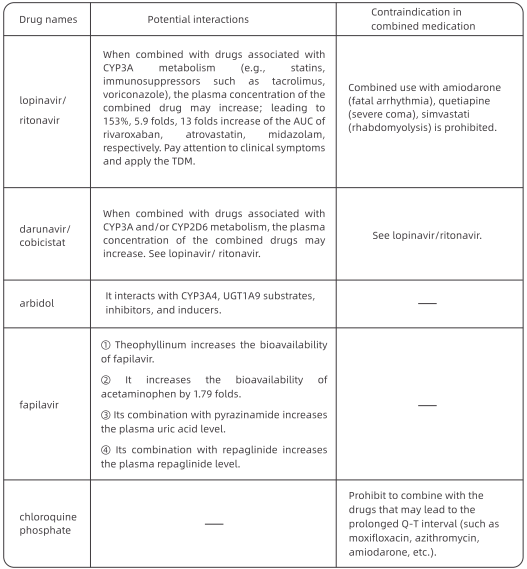
XIV. Psychological Intervention with COVID-19 Patients
1. The psychological stress and symptoms of COVID-19 patients Confirmed COVID-19 patients often have symptoms such as regret and resentment, loneliness and helplessness, depression, anxiety and phobia, irritation and sleep deprivation. Some patients may have panic attacks. Psychological evaluations in the isolated wards demonstrated that, about 48% of confirmed COVID-19 patients manifested psychological stress during early admission, most of which were from their emotional response to stress. The percentage of delirium is high among the critically ill patients. There is even a report of encephalitis induced by the SARS-CoV-2 leading to psychological symptoms such as unconsciousness and irritability.
2. Establishing a dynamic mechanism for evaluation and warning of psychological crisis Patients' mental states (individual psychological stress, mood, sleep quality, and pressure) should be monitored every week after admission and before discharge. The self-rating tools include: Self-Reporting Questionnaire 20 {SRQ-20), Patient Health Questionnaire 9 {PHQ-9) and Generalized Anxiety Disorder 7 {GAD-7). The peer-rating tools include: Hamilton Depression Rating Scale {HAMD), Hamilton Anxiety Rating Scale {HAMA), Positive and Negative Syndrome Scale (PANSS). In such a special environment as the isolated wards, we suggest that patients should be guided to complete the questionnaires through their cell phones. The doctors can interview and perform scale assessing through face-to-face or online discussion.
3. Intervention and treatment based on the assessment
3.1 Principles of intervention and treatment For mild patients, psychological intervention is suggested. Psychological self-adjustment includes breathing relaxation training and mindfulness training. For moderate to severe patients, intervention and treatment by combining medication and psychotherapy are suggested. New antidepressants, anxiolytics, and benzodiazepines can be prescribed to improve the patients' mood and sleep quality. The second generation antipsychotics such as olanzapine and quetiapine can be used to improve psychotic symptoms such as illusion and delusion.
3.2 The recommendation of psychotropic medications in elderly patients Middle-aged or elderly COVID-19 patients' medical situations are often complicated by physical diseases such as hypertension and diabetes. Therefore, when selecting psychotropic medications, the drug interactions and their effects on respiration must be fully considered. We recommend using citalopram, escitalopram, etc. to improve depression and anxiety symptoms; benzodiazepines such as estazolam, alprazolam, etc. to improve anxiety and sleep quality; olanzapine, quetiapine, etc. to improve psychotic symptoms.
XV. Rehabilitation Therapy for COVID-19 Patients
Severe and critically ill patients suffer from different degrees of dysfunction, especially respiratory insufficiency, dyskinesia and cognitive impairment, during both acute and recovery stages.
1. Rehabilitation therapy for severe and critically ill patients
The goal of early rehabilitation intervention is to reduce breathing difficulties, relieve symptoms, ease anxiety and depression and lower the incidence of complications. The process of early rehabilitation intervention is: rehabilitation assessment - therapy - reassessment.
1.1 Rehabilitation assessment
Based on general clinical assessment, especially functional evaluation, including respiration, cardiac status, motion and AOL should be emphasized. Focus on respiratory rehabilitation assessment, which includes the evaluation of thoracic activity, diaphragm activity amplitude, respiratory pattern and frequency, etc. 1.2 Rehabilitation therapy The rehabilitation therapy of severe or critically ill COVID-19 patients mainly includes position management, respiratory training, and physical therapy.
(1) Position management. Postural drainage may reduce the influence of sputum on the respiratory tract, which is especially important to improve the patient's V/Q. Patients must learn to tip themselves into a position which allows gravity to assist in draining excretion from lung lobes or lung segments. For patients using sedatives and suffering from consciousness disturbance, a standing-up bed or the bed head elevation (30°-45°-60°) may be applied if the patient's condition permits. Standing is the best body position for breathing in a resting state, which can effectively increase the patient's respiratory efficiency and maintain lung volume. As long as the patient feels good, let the patient take a standing position and gradually increase the time standing.
(2) Respiratory exercise. Exercise can fully expand the lungs, help the excretions from pulmonary alveoli and airway expel into the large airway so that sputum would not accumulate at the bottom of the lungs. It increases the vital capacity and enhances lung function. Deep-slow breathing and chest expansion breathing combined with shoulder expansion are the two major techniques of respiratory exercises.
① Deep-slow breathing: while inhaling, the patient should try his/her best to move the diaphragm actively. The breathing should be as deep and slow as possible to avoid the reduction of respiratory efficiency caused by fast-shallow breathing. Compared with thoracic breathing, this kind of breathing needs less muscle strength but has better tidal volume and V/Q value, which can be used to adjust breathing when experiencing short of breath. ② Chest expansion breathing combined with shoulder expansion: Increase pulmonary ventilation. When taking a deep-slow breath, one expands his/her chest and shoulders while inhaling; and moves back his/her chest and shoulders while exhaling. Due to the special pathological factors of viral pneumonia, suspending breathing for a long time should be avoided in order not to increase the burden of respiratory function, and the heart, as well as oxygen consumption. Meanwhile, avoid moving too fast. Adjust the respiratory rate at 12-15 times/min.
(3) Active cycle of breathing techniques. It can effectively remove bronchus excretion and improve lung function without exacerbation of hypoxemia and airflow obstruction. It consists of three stages (breathing control, thoracic expansion and exhalation). How to form a cycle of breathing should be developed according to the patient's condition.
(4) Positive expiratory pressure trainer. The pulmonary interstitium of COVID-19 patients has been severely damaged. In mechanical ventilation, low pressure and low tidal volume are required to avoid damages to the pulmonary interstitium. Therefore, after the removal of mechanical ventilation, positive expiratory pressure trainer can be used to help the movement of excretions from the low volume lung segments to the high-volume segments, lowering the difficulty of expectoration. Expiratory positive pressure can be generated through air flow vibration, which vibrates the airway to achieve airway supporting. The excretions can then be removed as the high-speed expiratory flow moves the excretions.
(5) Physical therapy. This includes ultrashort wave, oscillators, external diaphragm pacemaker, electrical muscle stimulation, etc.
XVI. Lung Transplantation in Patients with COVID-19
Lung transplantation is an effective treatment approach for final-stage chronic lung diseases. However, it is rarely reported that lung transplantation has been performed to treating acute infectious lung diseases. Based on current clinical practice and results, FAHZU summarized this chapter as a reference for medical workers. In general, following the principles of exploration, doing the best to save life, highly selective and high protection, if lung lesions are not significantly improved after adequate and reasonable medical treatment, and the patient is in critical condition, lung transplantation could be considered with other evaluations.
1. Pre-transplantation assessment
(1) Age: It is recommended that the recipients are not older than 70. Patients over 70 years old are subject to careful evaluation of other organ functions and postoperative recovery capability.
(2) The course of the disease: There is no direct correlation between the length of the disease course and the severity of the disease. However, for patients with short disease courses (fewer than 4-6 weeks), a full medical assessment is recommended to evaluate whether adequate medication, ventilator assistance, and ECMO support have been provided.
(3) Lung function status: Based on the parameters collected from lung CT, ventilator, and ECMO, it is necessary to evaluate whether there is any chance of recovery.
(4) Functional assessment of other major organs: a. Evaluation of the consciousness status of patients in critical condition using brain CT scan and electroencephalography is crucial, as most of them would have been sedated for an extended period; b. Cardiac assessments, including electrocardiogram and echocardiography that focus on right heart size, pulmonary artery pressure and left heart function, are highly recommended; c. The levels of serum creatinine and bilirubin should also be monitored; for patients with liver failure and renal failure, they should not be subjected to lung transplantation until the functions of the liver and kidney are recovered.
(5) The nucleic acid test of COVID-19: The patient should be tested negative for at least two consecutive nucleic acid tests with a time interval longer than 24 hours. Given the increased incidents of COVID-19 test result returning from negative to positive after treatment, it is recommended to revise the standard to three consecutive negative results. Ideally, negative results should be observed in all body fluid samples, including blood, sputum, nasopharynx, broncho-alveolar lavage, urine, and feces. Considering the difficulty in operation, however, at least the testing of sputum and broncho-alveolar lavage samples should be negative.
(6) Assessment of infection status: With the extended in-patient treatment, some COVID-19 patients may have multiple bacterial infections, and thus a full medical assessment is recommended to evaluate the situation of infection control, especially for multidrug-resistant bacterial infection. Moreover, post-procedure antibacterial treatment plans should be formed to estimate the risk of post-procedure infections.
(7) The preoperative medical assessment process for lung transplantation in COVID-19 patients: a treatment plan proposed by the ICU team • multidisciplinary discussion • comprehensive medical evaluation • analysis and treatment of relative contraindications • pre-habilitation before lung transplantation.
2. Contraindications Please refer to The 2014 ISHLT Consensus: A consensus document for the selection of lung transplantation candidates issued by the International Society for Heart and Lung Transplantation (updated in 2014).
XVII. Discharge Standards and Follow-up Plan for COVID-19 Patients
1. Discharge standards
(1) Body temperature remains normal for at least 3 days (ear temperature is lower than 37.5 °C);
(2) Respiratory symptoms are significantly improved;
(3) The nucleic acid is tested negative for respiratory tract pathogen twice consecutively (sampling interval more than 24 hours); the nucleic acid test of stool samples can be performed atthe same time if possible;
(4) Lung imaging shows obvious improvement in lesions;
(5) There is no comorbidities or complications which require hospitalization;
(6) SpO, > 93% without assisted oxygen inhalation;
(7) Discharge approved by multi-disciplinary medical team.
2. Medication after discharge Generally, antiviral drugs are not necessary after discharge. Treatments for symptoms can be applied if patients have mild cough, poor appetite, thick tongue coating, etc. Antiviral drugs can be used after discharge for patients with multiple lung lesions in the first 3 days after their nucleic acid are tested negative.
3. Home isolation Patients must continue two weeks of isolation after discharge. Recommended home isolation conditions are
① Independent living area with frequent ventilation and disinfection;
②Avoid contacting with infants, the elderly and people with weak immune functions at home;
③ Patients and their family members must wear masks and wash hands frequently;
④ Body temperature are taken twice a day (in the morning and evening) and pay close attention to any changes in the patient's condition.
4. Follow-up A specialized doctor should be arranged for each discharged patient's follow-ups. The first follow-up call should be made within 48 hours after discharge. The outpatient follow-up will be carried out 1 week, 2 weeks, and 1 month after discharge. Examinations include liver and kidney functions, blood test, nucleic acid test of sputum and stool samples, and pulmonary function test or lung CT scan should be reviewed according to the patient's condition. Follow-up phone calls should be made 3 and 6 months after discharge.
5. Management of patients tested positive again after discharge Strict discharge standards have been implemented in our hospital. There is no discharged case in our hospital whose sputum and stool samples are tested positive again in our follow-ups. However, there are some reported cases that patients are tested positive again, after being discharged based on the standards of national guidelines (negative results from at least 2 consecutive throat swabs collected at an interval of 24 hours; body temperature remaining normal for 3 days, symptoms significantly improved; obvious absorption of inflammation on lung images). It is mainly due to sample collection errors and false negative testing results. For these patients, the following strategies are recommended: {1) Isolation according to the standards for C0VID-19 patients. {2) Continuing to provide antiviral treatment which has been proved to be effective during prior hospitalization. (3) Discharge only when improvement is observed on lung imaging and the sputum and stool are tested negative for 3 consecutive times (with an interval of 24 hours). (4) Home isolation and follow-up visits after discharge in accordance with the requirements mentioned above.
VI. General Care
1. Monitoring Patient vital signs should be continuously monitored, especially changes in consciousness, respiration rate and the oxygen saturation. Observe symptoms such as cough, sputum, chest tightness, dyspnea, and cyanosis. Monitor arterial blood gas anlysis closely. Timely recognition of any deterioration to adjust strategies of oxygen therapy or to take urgent response measures. Pay attention to ventilator associated lung injury (VALi} when under high positive end-expiratory pressure (PEEP) and high-pressure support. Closely monitor changes in airway pressure, tidal volume and respiratory rate.
2.Aspiration Prevention
(1) Gastric retention monitor: perform continuous post-pyloric feeding with a nutrition pump to reduce gastroesophageal reflux. Evaluate gastric motility and gastric retention with ultrasound if possible. Patient with normal gastric emptying are not recommended for routine assessment;
(2) Evaluate gastric retention every 4 hours. Re-infuse the aspirate if the gastric residual volume is< 100 ml; otherwise, report to the attending physician;
(3) Aspiration prevention during patient transportation: before transportation, stop nasal feeding, aspirate the gastric residues and connect the gastric tube to a negative pressure bag. During transportation, raise the patient's head up to 30°;
(4) Aspiration prevention during HFNC: Check the humidifier every 4 hours to avoid excessive or insufficient humidification. Remove any water accumulated in the tubing promptly to prevent cough and aspiration caused by the accidental entry of condensation into the airway. Keep the position of the nasal cannula higher than the machine and tubes. Promptly remove condensation in the system.

